Question: Construct an approximate boiling-point-composition diagram for a benzene methanol system. The mixture shows azeotropic behavior (see Table 15.3). Include on the graph the boiling points
Construct an approximate boiling-point-composition diagram for a benzene– methanol system. The mixture shows azeotropic behavior (see Table 15.3). Include on the graph the boiling points of pure benzene and pure methanol and the boiling point of the azeotrope. Describe the behavior on distillation of a mixture that is initially rich in benzene (90%) and then for a mixture that is initially rich in methanol (90%).
(Reference Table 15.3)
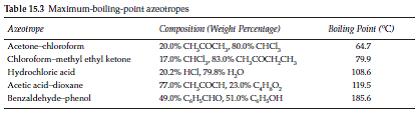
Table 15.3 Maximum-boling-polint azeotropes Azeotrope Compositisn (Weight Percentage) Boiling Point (C) 20.0% CHCOCH, 80.0% CHCI, 17.0% CHCI, 30%CHCOCHCH, 20.2% HCI, 79.8% H,0 77.% CH COCH, 23.0% CH0, 49.0% CHCHO, 51.0% CH,OH Acetone-chloroform 64.7 Chlorofoem-methyl ethyl ketone Hydrochlorie acid Acetic acid-dioxane 79.9 108.6 119.5 Benzaldehyde phenol 185.6
Step by Step Solution
3.38 Rating (160 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


