Question: Continuation from previous task: Use le Chatelier's principle to determine what happens to the following equilibrium of the given changes under When some hydrogen gas
Continuation from previous task:
Use le Chatelier's principle to determine what happens to the following equilibrium of the given changes under
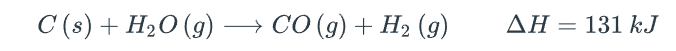
When some hydrogen gas is removed, the equilibrium will be shifted towards
When something C(s) is added, the equilibrium will be shifted towards the
When more H2O(g) is added, the equilibrium will be shifted towards
When the pressure increases by reducing the volume (assuming the temperature is kept constant), the equilibrium will be shifted towards the
When the gas mixture cools, the equilibrium will be shifted towards the
When a catalyst is added
C(s) + H2O (9) + CO (9) + H2 (9) C s)+ (g) ) = 131 kJ
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


