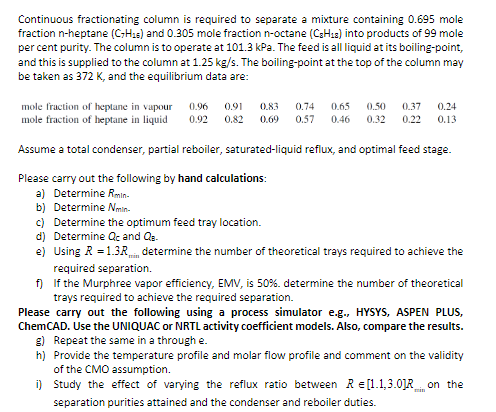
Question: Continuous fractionating column is required to separate a mixture containing 0 . 6 9 5 mole fraction n - heptane ( C 7 H 1
Continuous fractionating column is required to separate a mixture containing mole
fraction heptane and mole fraction octane into products of mole
per cent purity. The column is to operate at kPa. The feed is all liquid at its boilingpoint,
and this is supplied to the column at The boilingpoint at the top of the column may
be taken as and the equilibrium data are:
Assume a total condenser, partial reboiler, saturatedliquid reflux, and optimal feed stage.
Please carry out the following by hand calculations:
a Determine
b Determine
c Determine the optimum feed tray location.
d Determine and
e Using determine the number of theoretical trays required to achieve the
required separation.
f If the Murphree vapor efficiency, EMV, is determine the number of theoretical
trays required to achieve the required separation.
Please carry out the following using a process simulator eg HYSYS ASPEN PLUS,
ChemCAD. Use the UNIQUAC or NRTL activity coefficient models. Also, compare the results.
g Repeat the same in a through e
h Provide the temperature profile and molar flow profile and comment on the validity
of the CMO assumption.
i Study the effect of varying the reflux ratio between Rin on the
separation purities attained and the condenser and reboiler duties.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


