Question: Convert 5.85 g/L into g/100ML (note: 100 ml = 0.1L) MW = 58.5 g/mol 5.85% = 5.85%00om 5.85g 1000ml 100ml X= 0.59% What percentage
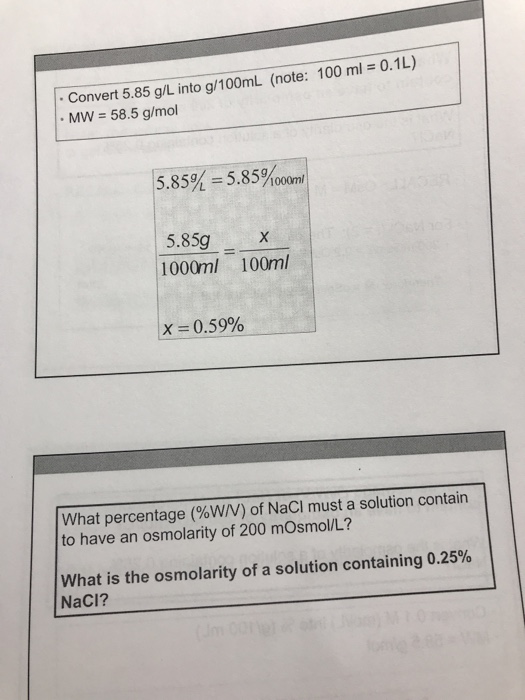
Convert 5.85 g/L into g/100ML (note: 100 ml = 0.1L) MW = 58.5 g/mol 5.85% = 5.85%00om 5.85g 1000ml 100ml X= 0.59% What percentage (%W/V) of NaCl must a solution contain to have an osmolarity of 200 mOsmol/L? What is the osmolarity of a solution containing 0.25% NaCl? (Jm 00
Step by Step Solution
3.31 Rating (148 Votes )
There are 3 Steps involved in it
formula 1 Osmol L W V x 10 ... View full answer

Get step-by-step solutions from verified subject matter experts


