Question: The weak base ethylamine (CH5NH (aq)) has a K of 6.4 x104. a. Write the equilibrium equation for the ionization of ethylamine. b. What
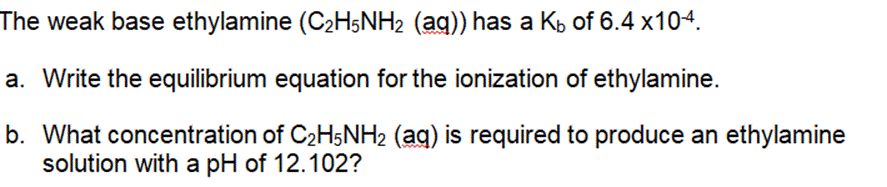
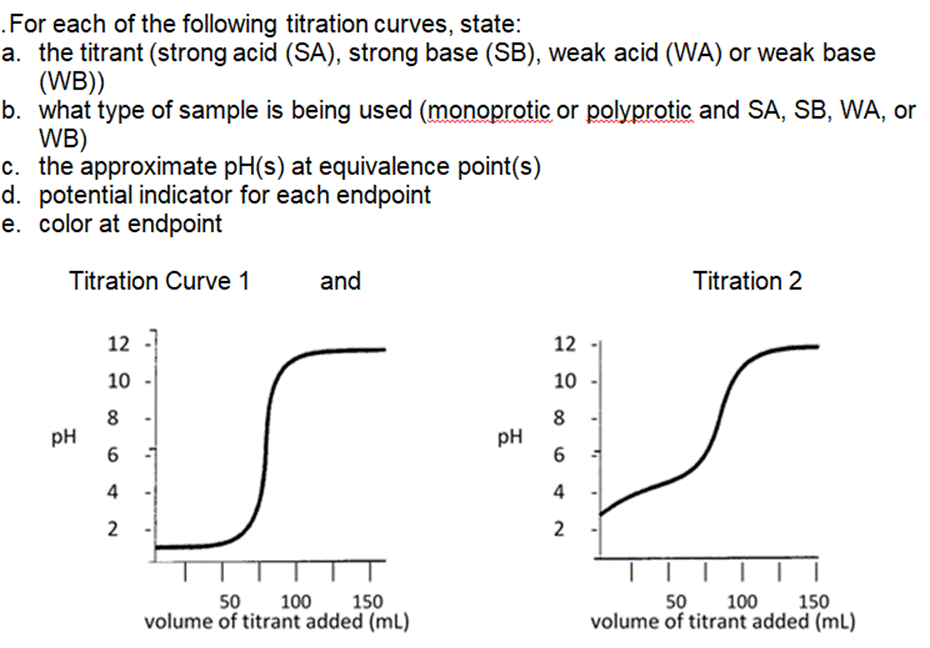
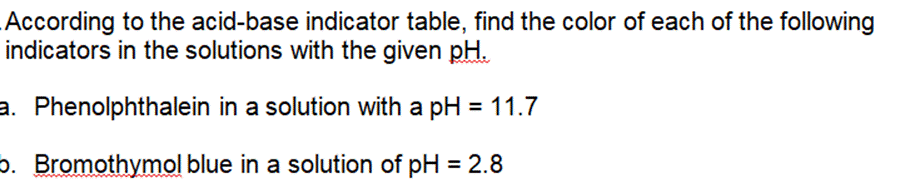
The weak base ethylamine (CH5NH (aq)) has a K of 6.4 x104. a. Write the equilibrium equation for the ionization of ethylamine. b. What concentration of CH5NH2 (aq) is required to produce an ethylamine solution with a pH of 12.102? .For each of the following titration curves, state: a. the titrant (strong acid (SA), strong base (SB), weak acid (WA) or weak base (WB)) b. what type of sample is being used (monoprotic or polyprotic and SA, SB, WA, or WB) c. the approximate pH(s) at equivalence point(s) d. potential indicator for each endpoint e. color at endpoint Titration Curve 1 PH 12 10 8 6 4 2 and v T T 50 150 100 volume of titrant added (ml) PH 12 10 8 6 4 2 Titration 2 50 100 150 volume of titrant added (ml) According to the acid-base indicator table, find the color of each of the following indicators in the solutions with the given pH. a. Phenolphthalein in a solution with a pH = 11.7 . Bromothymol blue in a solution of pH = 2.8
Step by Step Solution
There are 3 Steps involved in it
SOLUTION a The equilibrium equation for the ionization of ethylamine C2H5NH2 can be written as follows C2H5NH2 aq H2O l C2H5NH3 aq OH aq In this equat... View full answer

Get step-by-step solutions from verified subject matter experts


