Question: cording to Exercise 3.4: Temperature effect on reaction equilibria Hexane can equilibrate to methyl-substituted pentanes and methylcyclopentane ac- ky hexane methylcyclopentane + H2 k-1 k2
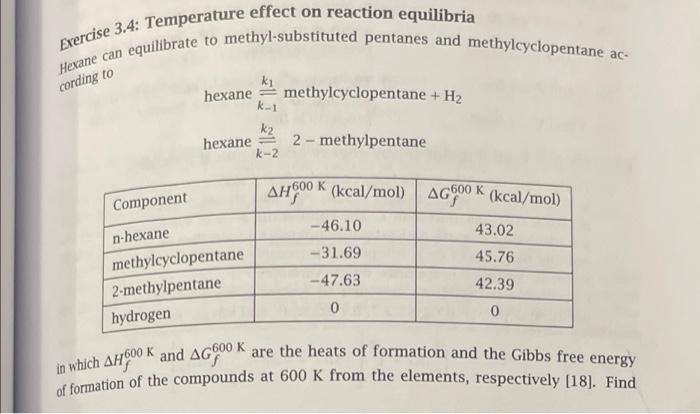
cording to Exercise 3.4: Temperature effect on reaction equilibria Hexane can equilibrate to methyl-substituted pentanes and methylcyclopentane ac- ky hexane methylcyclopentane + H2 k-1 k2 hexane 2-methylpentane k-2 AHOK (kcal/mol) AG600 K (kcal/mol) -46.10 43.02 -31.69 45.76 -47.63 42.39 0 0 Component n-hexane methylcyclopentane 2-methylpentane hydrogen K in which AHOOO and AG600 K are the heats of formation and the Gibbs free energy of formation of the compounds at 600 K from the elements, respectively [18]. Find 100 1 T.P, nj 2 T, P,Bn Figure 3.7: Two systems with identical intensive properties and dif- ferent extensive properties. the temperature where equal amounts of methylcyclopentane and 2-methylpentant form if pure hexane is allowed to equilibrate at 1.0 atm total pressure. Also find the composition of the mixture at this temperature. At this condition the hydrocarbons are gases
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


