Question: Problem 3.4 below: Biven 0 (partial answer. Exercise 3.4: Temperature effect on reaction equilibria Hexane can equilibrate to methyl-substituted pentanes and methylcyclopentane ac- cording to
Problem 3.4 below:
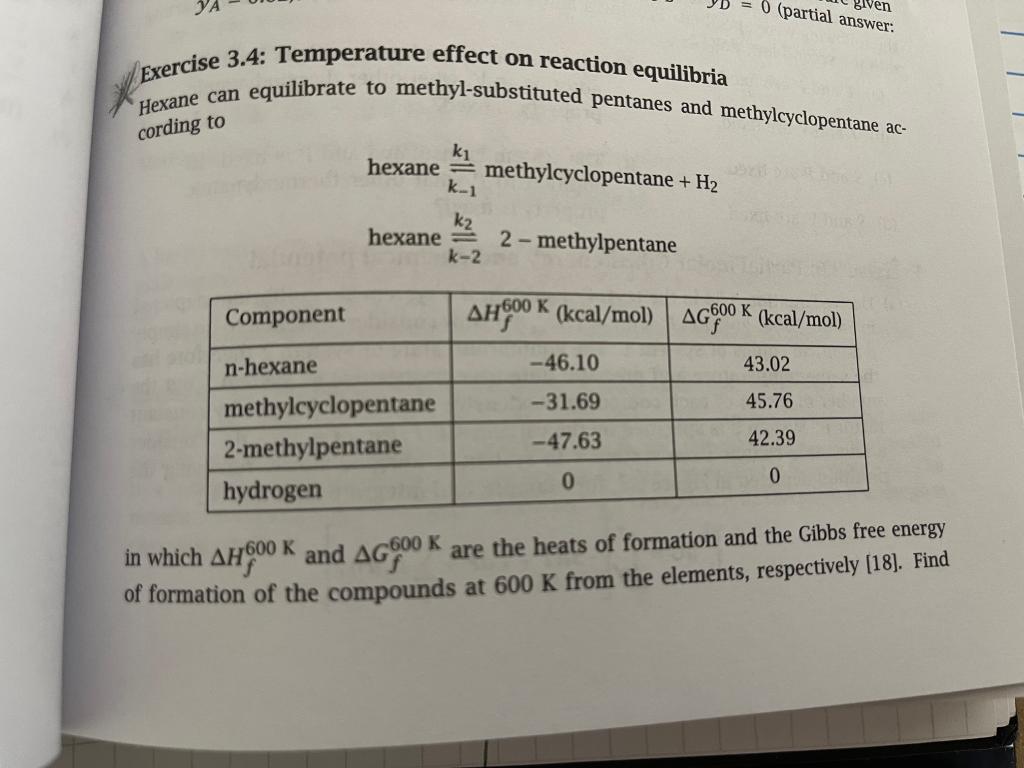
Biven 0 (partial answer. Exercise 3.4: Temperature effect on reaction equilibria Hexane can equilibrate to methyl-substituted pentanes and methylcyclopentane ac- cording to hexane = methylcyclopentane + H2 k-1 k2 hexane k-2 2-methylpentane Component AHSO0 K (kcal/mol) AG900 K (kcal/mol) 600 n-hexane -46.10 43.02 -31.69 45.76 methylcyclopentane 2-methylpentane hydrogen -47.63 42.39 0 0 -600 in which AHoo K and AG OOK are the heats of formation and the Gibbs free energy of formation of the compounds at 600 K from the elements, respectively (18]. Find Review of Chemical Equilibrium 3.7 Exercis 100 Beca wit an 1 T, P, nj (b) S di sisse - DOBROTESTELD doll 2 T,P, Bn; COTTOM Figure 3.7: Two systems with identical intensive properties and dif- ferent extensive properties. { Exe Co the temperature where equal amounts of methylcyclopentane and 2-methylpentane form if pure hexane is allowed to equilibrate at 1.0 atm total pressure. Also find the composition of the mixture at this temperature. At this condition the hydrocarbons are gases
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


