Question: CORROSION ENGINEERING. PLEASE ANSWER A AND B CORRECTLY WITH STEPS. I WILL LEAVE A GOOD RATING IMMEDIATELY! Problem 2: Rate Constants for High Temperature Corrosion
CORROSION ENGINEERING. PLEASE ANSWER A AND B CORRECTLY WITH STEPS. I WILL LEAVE A GOOD RATING IMMEDIATELY!
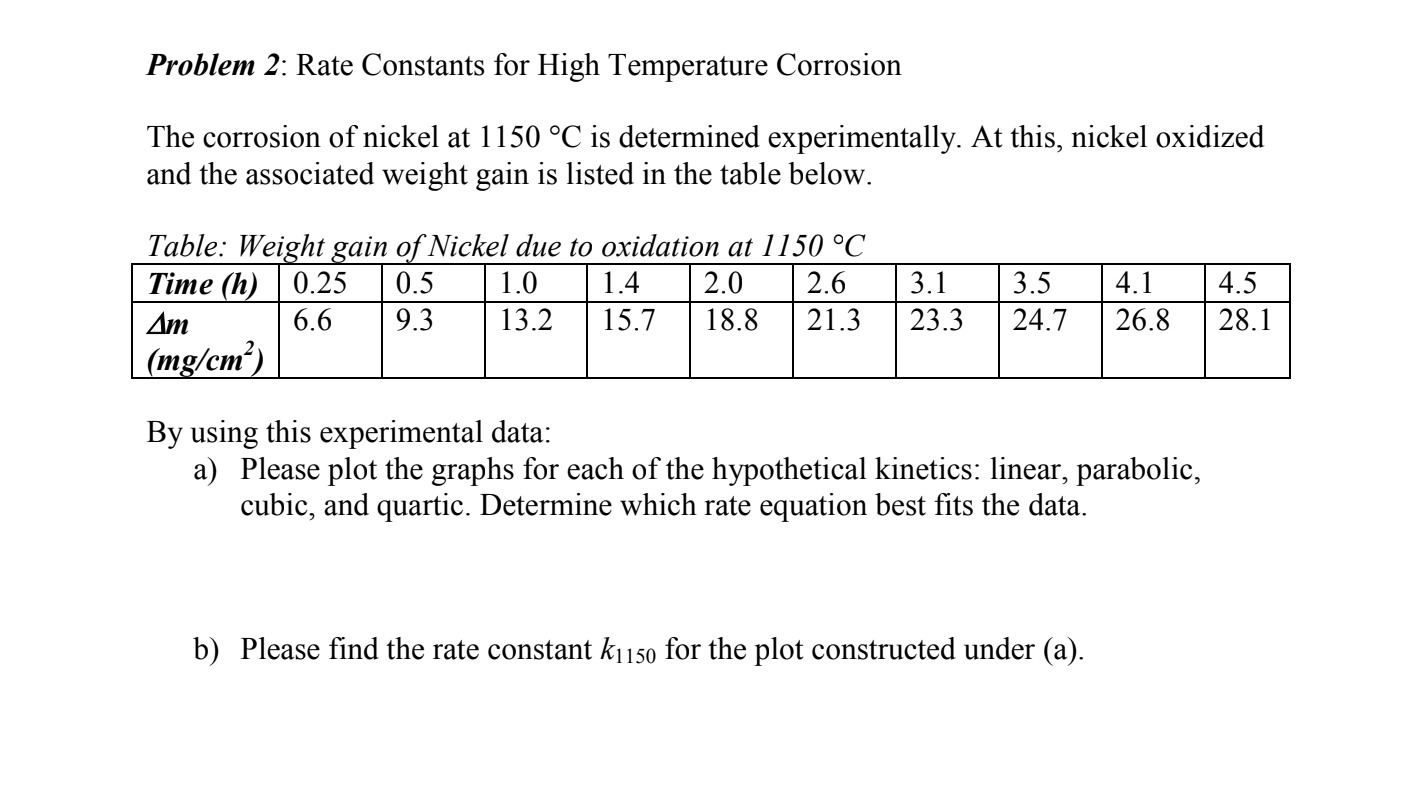
Problem 2: Rate Constants for High Temperature Corrosion The corrosion of nickel at 1150C is determined experimentally. At this, nickel oxidized and the associated weight gain is listed in the table below. Table: Weight gain of Nickel due to oxidation at 1150C By using this experimental data: a) Please plot the graphs for each of the hypothetical kinetics: linear, parabolic, cubic, and quartic. Determine which rate equation best fits the data. b) Please find the rate constant k1150 for the plot constructed under (a)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


