Question: could i please grab some help with this question, thank you An acetic acid/acetate buffer was created by starting with a solution of acetic acid.
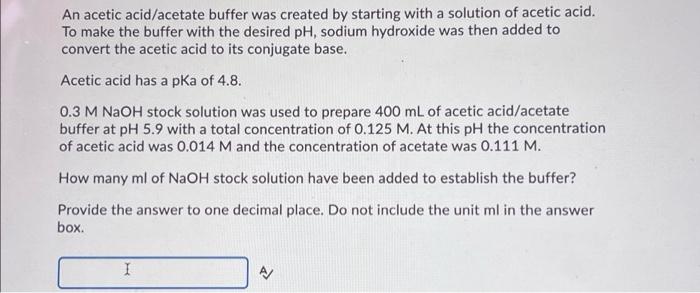
An acetic acid/acetate buffer was created by starting with a solution of acetic acid. To make the buffer with the desired pH, sodium hydroxide was then added to convert the acetic acid to its conjugate base. Acetic acid has a pKa of 4.8. 0.3 M NaOH stock solution was used to prepare 400 mL of acetic acid/acetate buffer at pH 5.9 with a total concentration of 0.125 M. At this pH the concentration of acetic acid was 0.014 M and the concentration of acetate was 0.111 M. How many ml of NaOH stock solution have been added to establish the buffer? Provide the answer to one decimal place. Do not include the unit ml in the answer box
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


