Question: Please help with number 2 & 3 LabQuest 19 12. Use a graduated cylinder to measure out 10.0 mL of the Buffer B solution and
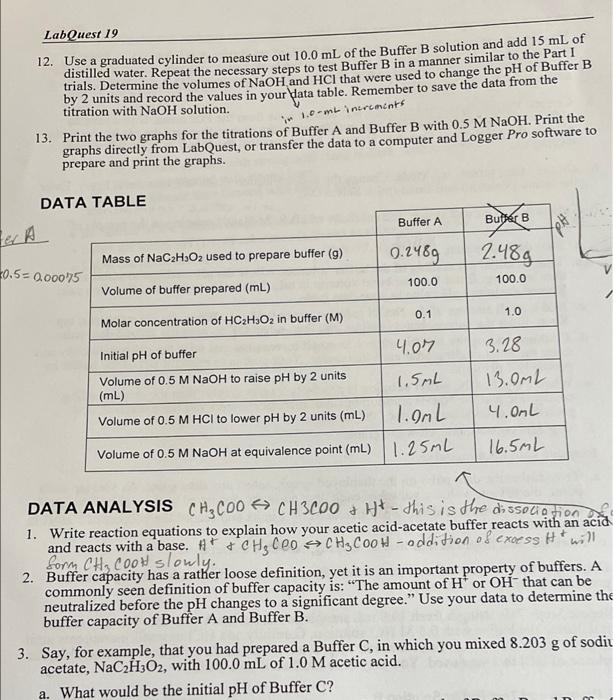
LabQuest 19 12. Use a graduated cylinder to measure out 10.0 mL of the Buffer B solution and add 15 mL of distilled water. Repeat the necessary steps to test Buffer B in a manner similar to the Part I trials. Determine the volumes of NaOH and HCl that were used to change the pH of Buffer B by 2 units and record the values in your data table. Remember to save the data from the titration with NaOH solution. 13. Print the two graphs for the titrations of Buffer A and Buffer B with 0.5 M NaOH. Print the graphs directly from LabQuest, or transfer the data to a computer and Logger Pro software to prepare and print the graphs. 1.combinerements DATA TABLE Buffer A Butter B Hd tel Mass of NaC2H2O2 used to prepare buffer (9) 0.2489 2.48g V 40.5= 0.00075 100.0 100.0 Volume of buffer prepared (mL) 0.1 1.0 Molar concentration of HC2H,O2 in buffer (M) Initial pH of buffer 4.07 Volume of 0.5 M NaOH to raise pH by 2 units 1.5ML (mL) Volume of 0.5 M HCl to lower pH by 2 units (mL) 1.OML Volume of 0.5 M NaOH at equivalence point (mL) 1.25ML 3.28 13.0ML 4.00L 16.5ML a DATA ANALYSIS CH3C00 CH3C00+ H+ - this is the dissociation 1. Write reaction equations to explain how your acetic acid-acetate buffer reacts with an acid and reacts with a base. #*+CH, CO CHCOOH - oddition of excess H* will form CH, CO0W slowly. 2. Buffer capacity has a rather loose definition, yet it is an important property of buffers. A commonly seen definition of buffer capacity is: "The amount of H' or OH- that can be neutralized before the pH changes to a significant degree." Use your data to determine the buffer capacity of Buffer A and Buffer B. 3. Say, for example, that you had prepared a Buffer C, in which you mixed 8.203 g of sodiu acetate, NaCH3O2, with 100.0 mL of 1.0 M acetic acid. a. What would be the initial pH of Buffer C
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


