Question: Could someone help me write an abstract for a lab report and maybe give some helpful writing tips? For this assignment, the target compound that
Could someone help me write an abstract for a lab report and maybe give some helpful writing tips?
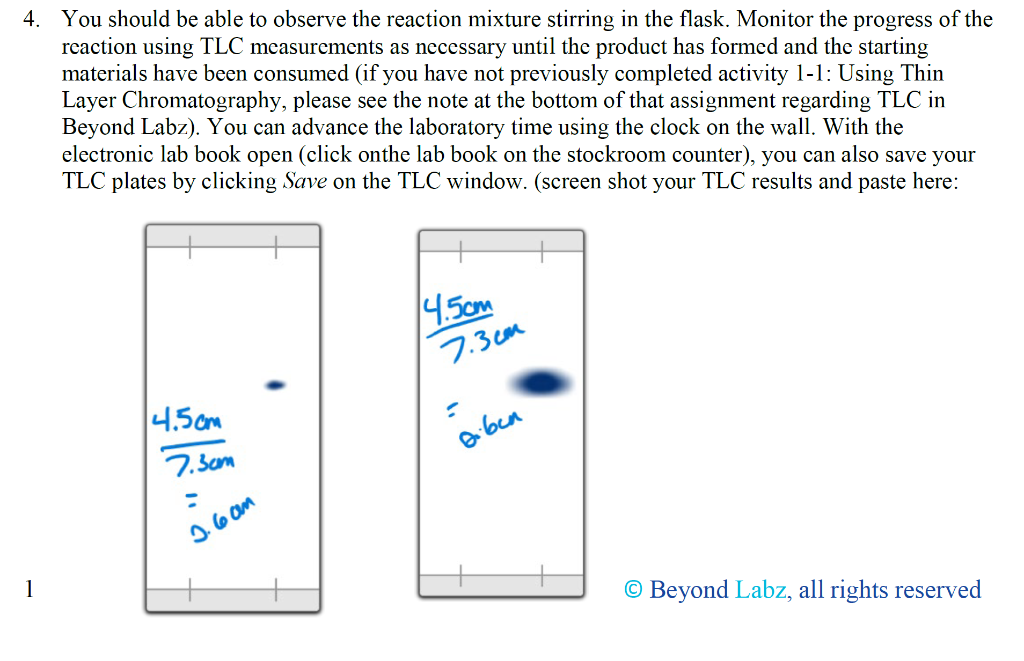
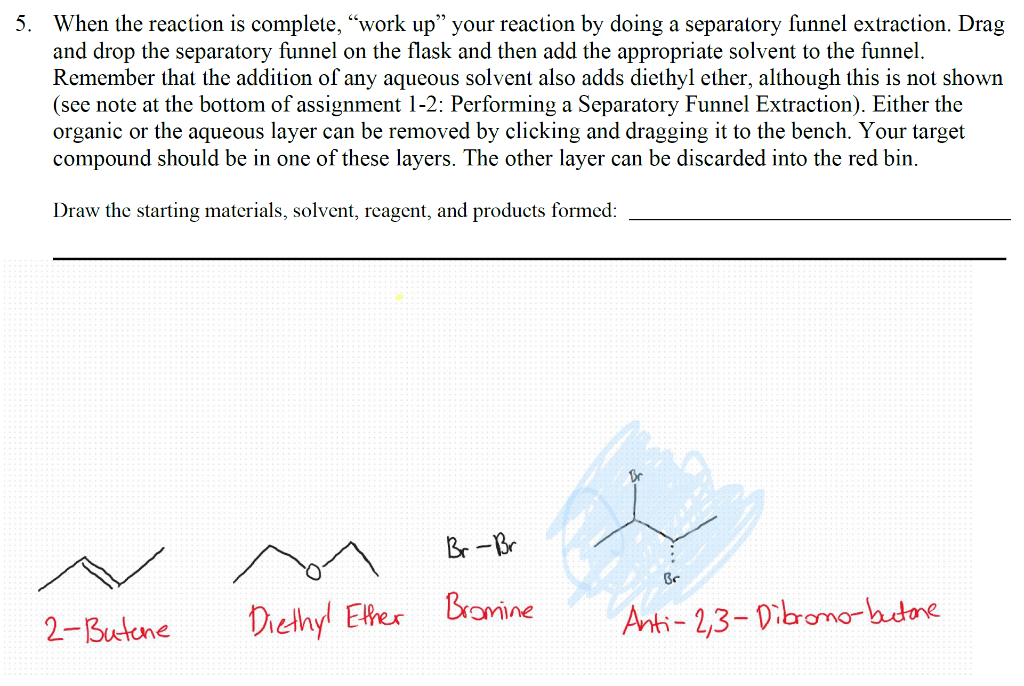
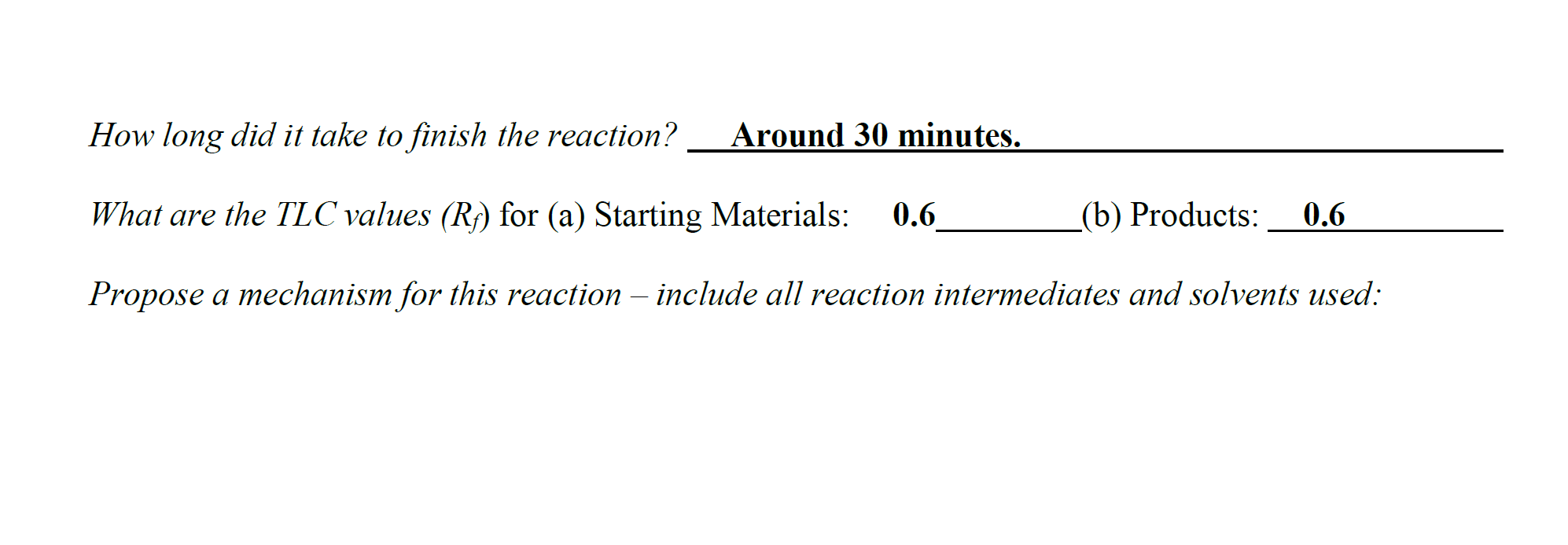
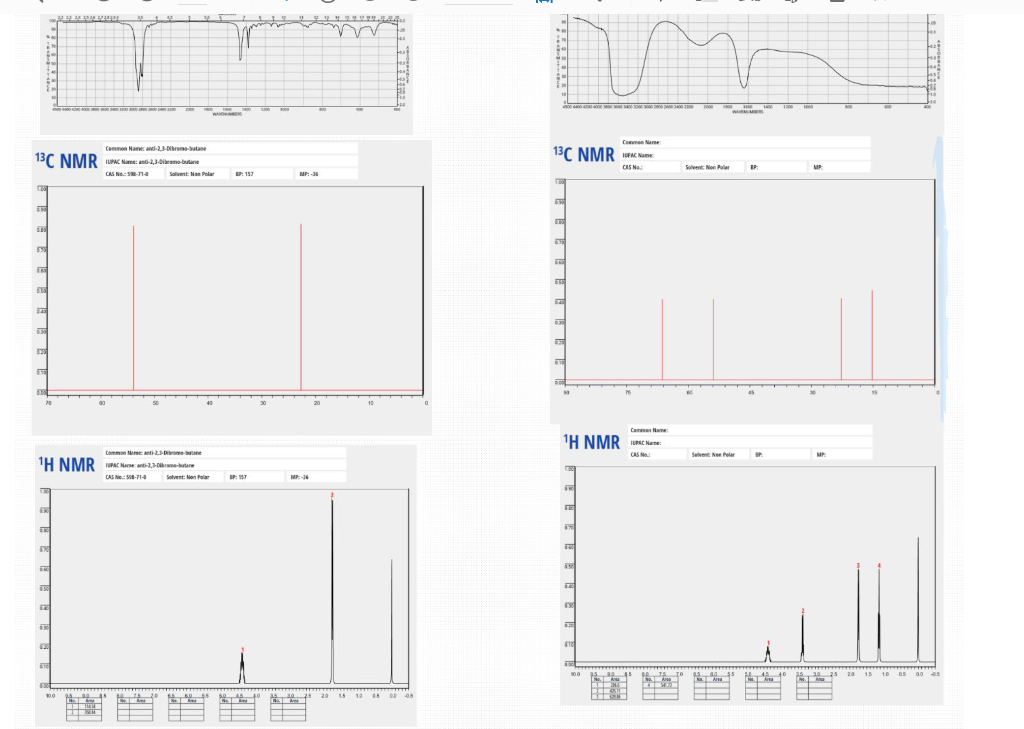
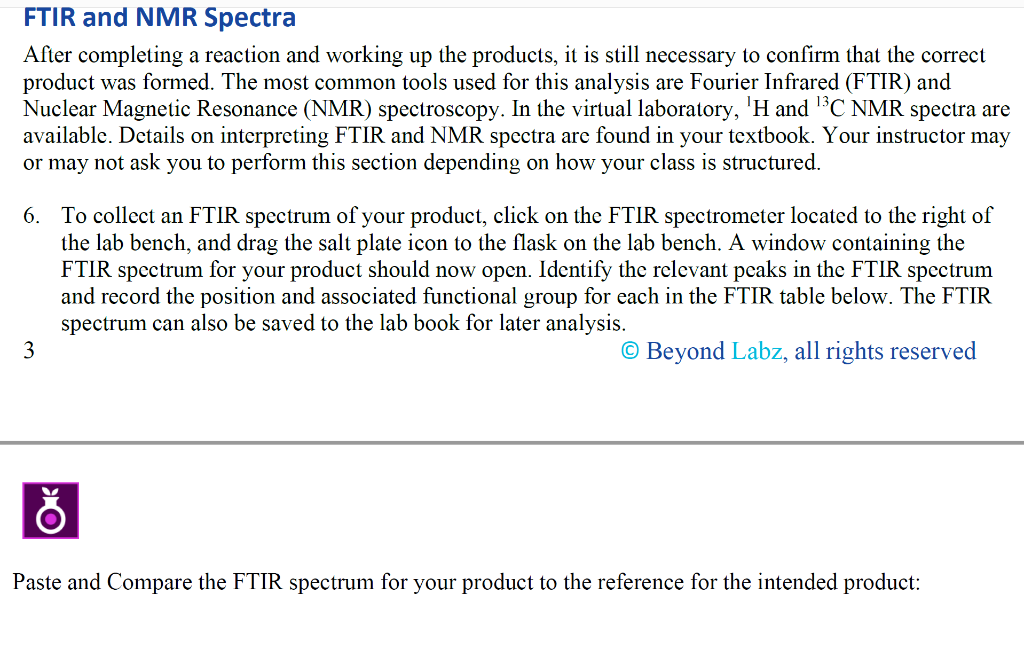


For this assignment, the target compound that you should synthesize is anti-2,3-dibromo-butane. This is a stereospecific electrophilic alkene addition reaction. Examine the product to determine the location of the new functionality. Keep in mind the bromonium ion intermediate and the consequences of its structure. The nucleophile again attacks in a manner that controls the stereochemistry of the product. Synthesis Procedures 1. Start Virtual ChemLab Organic and select Alkene Bromination - 1 from the list of assignments. After entering the synthesis laboratory use the available reagents on the stockroom shelf, identify the appropriate starting materials required to synthesize the target compound and add them to the round bottom flask. Now add ether (Et2O) as a solvent and drag the flask to the Stir Plate on the lab bench. Why do we use diethyl ether for the solvent in this reaction? Like other ethers, diethyl ether is a preferred solvent due to its non-polarity. 2. The round bottom flask containing the starting materials should now be on the stir plate, and the contents of the flask should be visible on the chalkboard. From the group of reagents found on the lab bench, select the correct reagent to synthesize the target compound and add it to the flask on the stir plate. 3. Start the reaction by clicking on the Stir button on the front of the stir plate. - Why don't we need HEAT in this reaction? What are the typical reaction conditions for this type of electrophilic halogen addition? 2. The round bottom flask containing the starting materials should now be on the stir plate, and the contents of the flask should be visible on the chalkboard. From the group of reagents found on the lab bench, select the correct reagent to synthesize the target compound and add it to the flask on the stir plate. 3. Start the reaction by clicking on the Stir button on the front of the stir plate. - Why don't we need HEAT in this reaction? What are the typical reaction conditions for this type of electrophilic halogen addition? Dutside heat sources aren't needed due to its endothermic nature and enthalpy change. 4. You should be able to observe the reaction mixture stirring in the flask. Monitor the progress of the reaction using TLC measurements as necessary until the product has formed and the starting materials have been consumed (if you have not previously completed activity 1-1: Using Thin Layer Chromatography, please see the note at the bottom of that assignment regarding TLC in Beyond Labz). You can advance the laboratory time using the clock on the wall. With the electronic lab book open (click onthe lab book on the stockroom counter), you can also save your TLC plates by clicking Save on the TLC window. (screen shot your TLC results and paste here: You should be able to observe the reaction mixture stirring in the flask. Monitor the progress of the reaction using TLC measurements as necessary until the product has formed and the starting materials have been consumed (if you have not previously completed activity 1-1: Using Thin Layer Chromatography, please see the note at the bottom of that assignment regarding TLC in Beyond Labz). You can advance the laboratory time using the clock on the wall. With the electronic lab book open (click onthe lab book on the stockroom counter), you can also save your TLC plates by clicking Save on the TLC window. (screen shot your TLC results and paste here: Beyond Labz, all rights reserved 5. When the reaction is complete, "work up" your reaction by doing a separatory funnel extraction. Drag and drop the separatory funnel on the flask and then add the appropriate solvent to the funnel. Remember that the addition of any aqueous solvent also adds diethyl ether, although this is not shown (see note at the bottom of assignment 1-2: Performing a Separatory Funnel Extraction). Either the organic or the aqueous layer can be removed by clicking and dragging it to the bench. Your target compound should be in one of these layers. The other layer can be discarded into the red bin. Draw the starting materials, solvent, reagent, and products formed: How long did it take to finish the reaction? Around 30 minutes. What are the TLC values (Rf) for (a) Starting Materials: 0.6 (b) Products: Propose a mechanism for this reaction - include all reaction intermediates and solvents used: After completing a reaction and working up the products, it is still necessary to confirm that the correct product was formed. The most common tools used for this analysis are Fourier Infrared (FTIR) and Nuclear Magnetic Resonance (NMR) spectroscopy. In the virtual laboratory, 1H and 13C NMR spectra are available. Details on interpreting FTIR and NMR spectra are found in your textbook. Your instructor may or may not ask you to perform this section depending on how your class is structured. 6. To collect an FTIR spectrum of your product, click on the FTIR spectrometer located to the right of the lab bench, and drag the salt plate icon to the flask on the lab bench. A window containing the FTIR spectrum for your product should now open. Identify the relevant peaks in the FTIR spectrum and record the position and associated functional group for each in the FTIR table below. The FTIR spectrum can also be saved to the lab book for later analysis. 3 Beyond Labz, all rights reserved Paste and Compare the FTIR spectrum for your product to the reference for the intended product: Be sure to include all TLC strips and spectroscopy scans when showing final products vs targeted molecules. 1) Which starting reagent did you use? 2) Draw a mechanism for this reaction. 3) Are protic solvents effective at stabilizing ions? 4) Are SN1 or SN2 favored utilizing a polar protic solvent? 5) From the red cap containers, which additional reagent was utilized? 6) Why do we utilize ether as the solvent (solvent is located in the top of the stock room content on the right)? How would the reaction be different if we included a non-polar solvent instead? 7) Why don't we need heat for this reaction (consider enthalpy of formation) - provide a calculation and determine if this is an exothermic or endothermic process. 8) Compare the TLC 1-2 minutes after the reaction has started. Why does the spot on the right (products) seem to split? What does the new dot represent in terms of polarity of the new product? Use rf values and the properties of TLC to justify your answer. 9) During our separation/extraction, what layer did the product end up in? How would the solvent be removed to isolate the product in the real world? 10) Does the reference FTIR scan for the product match what you extracted? For this assignment, the target compound that you should synthesize is anti-2,3-dibromo-butane. This is a stereospecific electrophilic alkene addition reaction. Examine the product to determine the location of the new functionality. Keep in mind the bromonium ion intermediate and the consequences of its structure. The nucleophile again attacks in a manner that controls the stereochemistry of the product. Synthesis Procedures 1. Start Virtual ChemLab Organic and select Alkene Bromination - 1 from the list of assignments. After entering the synthesis laboratory use the available reagents on the stockroom shelf, identify the appropriate starting materials required to synthesize the target compound and add them to the round bottom flask. Now add ether (Et2O) as a solvent and drag the flask to the Stir Plate on the lab bench. Why do we use diethyl ether for the solvent in this reaction? Like other ethers, diethyl ether is a preferred solvent due to its non-polarity. 2. The round bottom flask containing the starting materials should now be on the stir plate, and the contents of the flask should be visible on the chalkboard. From the group of reagents found on the lab bench, select the correct reagent to synthesize the target compound and add it to the flask on the stir plate. 3. Start the reaction by clicking on the Stir button on the front of the stir plate. - Why don't we need HEAT in this reaction? What are the typical reaction conditions for this type of electrophilic halogen addition
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


