Question: could you please also help and explain problems 4 and 5? im confused on it and need clarifications 4) An equilibrium mixture of NO2 and
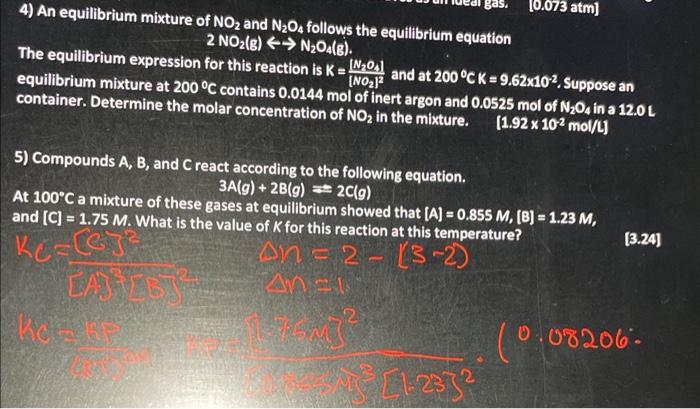
4) An equilibrium mixture of NO2 and N2O4 follows the equilibrium equation The equilibrium expression for this reaction is K=[NO2]2[N2O3] and at 200CK=9.62102. Suppose an equilibrium mixture at 200C contains 0.0144mol of inert argon and 0.0525 mol of N2O4 in a 12.0L container. Determine the molar concentration of NO2 in the mixture. [1.92102mol/L] 5) Compounds A, B, and C react according to the following equation. At 100C a mixture of these gases at equilibrium showed that [A]=0.855M,[B]=1.23M, and [C]=1.75M. What is the value of K for this reaction at this temperature? KC=(G)2A)=2(32) [3.24] (0.08206
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


