Question: 1. Crystallization paths, heating paths, quantitative calculations and vertical sections for abstract systems: 1. Using Diagram PS.1, draw joins which will create the proper composition
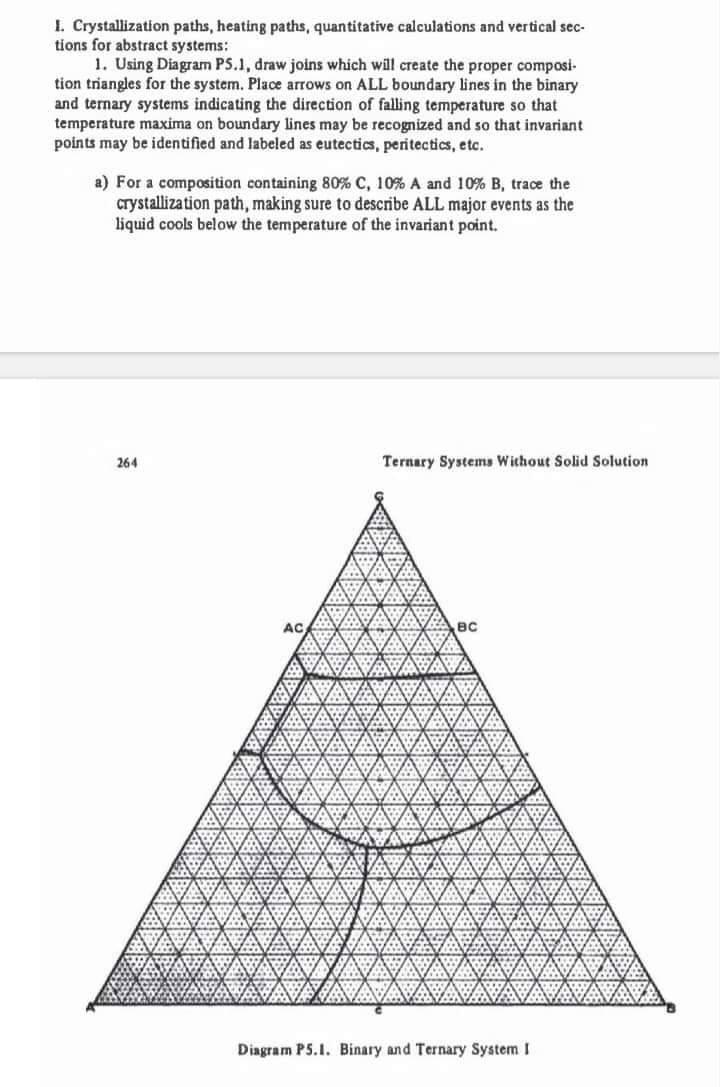

1. Crystallization paths, heating paths, quantitative calculations and vertical sections for abstract systems: 1. Using Diagram PS.1, draw joins which will create the proper composition triangles for the system. Place arrows on ALL boundary lines in the binary and ternary systems indicating the direction of falling temperature so that temperature maxima on boundary lines may be recognized and so that invariant points may be identified and labeled as eutectics, peritectics, etc. a) For a composition containing 80%C,10%A and 10%B, trace the crystallization path, making sure to describe ALL major events as the liquid cools below the temperature of the invariant point. 264 Termary Systems Without Solid Solution Diagram P5.1. Binary and Ternary System I b) Show QUANITTATIVELY by calculation that one phase partially dis. solves at the invariant point as heat is withdrawn from the system. c) Show that the amount of A,B, and C in the final crystallized mixture is the same as that in the original liquid. (80C,10A,10B) d) Trace the crystallization path of a composition containing 75%C,15% A and 10%B. The composition is on the join ACBC. Make a quantitative calculation just before and just after final crystallization to show exactly what takes place at the invariant point. e) Trace the crystallization path of a liquid containing 65%C,20%A and 15%B on the boundary line between the primary fields of C and BC. Problems 265 f) How much solid and how much liquid coexist when the liquid composition is 60%C,30%A and 10%B ? What is the composition of the solid? g) Make a construction which shows the area in which starting liquids in the primary field of C and in the composition triangle ACBCA will cross the primary field of BC as they proceed to the eutectic where AC, BC and A coexist. h) For the liquid composition given in (e) above, make a quantitative analysis of the equilibrium just before and just after final crystallization at the eutectic. Show that the amount of A,B, and C in the assemblage of liquid, AC and BC just before final crystallization is the same as that in the starting liquid (65C,20A,15B). Show that the amount of A,B and C in the final crystallized mixture of A,AC and BC is the same as that in the starting liquid (65C, 20A, 15B). How much A is precipitated during final crystallization at the invariant point? i) Trace the crystallization path of a liquid containing 70%C,5%A and 25\% B. Be sure to describe ALL major events which occur as the temperature is lowered below the invariant point. Make quantitative calculations at temperatures where C is in equilibrium with liquid, where C and BC are in equilibrium with Hquld, where only BC is in equilibrium with liquid, where BC and A are in equilibrium with liquid and just before and just after final crystallization at the invariant point. How much B is precipitated during reaction at the invariant point? j) Draw the vertical section for the Join AC-BC. Assume reasonable temperature relationships for the system and label each area of the diagram for phases present. Use a quantitative scale for the horizontal composition axis. k) Draw the vertical section between C-c. Assume reasonable temperature relationships in the system and label each area of the diagram for phases present. Make sure the horizontal composition axis is reasonably quan titative between C and c. 1. Crystallization paths, heating paths, quantitative calculations and vertical sections for abstract systems: 1. Using Diagram PS.1, draw joins which will create the proper composition triangles for the system. Place arrows on ALL boundary lines in the binary and ternary systems indicating the direction of falling temperature so that temperature maxima on boundary lines may be recognized and so that invariant points may be identified and labeled as eutectics, peritectics, etc. a) For a composition containing 80%C,10%A and 10%B, trace the crystallization path, making sure to describe ALL major events as the liquid cools below the temperature of the invariant point. 264 Termary Systems Without Solid Solution Diagram P5.1. Binary and Ternary System I b) Show QUANITTATIVELY by calculation that one phase partially dis. solves at the invariant point as heat is withdrawn from the system. c) Show that the amount of A,B, and C in the final crystallized mixture is the same as that in the original liquid. (80C,10A,10B) d) Trace the crystallization path of a composition containing 75%C,15% A and 10%B. The composition is on the join ACBC. Make a quantitative calculation just before and just after final crystallization to show exactly what takes place at the invariant point. e) Trace the crystallization path of a liquid containing 65%C,20%A and 15%B on the boundary line between the primary fields of C and BC. Problems 265 f) How much solid and how much liquid coexist when the liquid composition is 60%C,30%A and 10%B ? What is the composition of the solid? g) Make a construction which shows the area in which starting liquids in the primary field of C and in the composition triangle ACBCA will cross the primary field of BC as they proceed to the eutectic where AC, BC and A coexist. h) For the liquid composition given in (e) above, make a quantitative analysis of the equilibrium just before and just after final crystallization at the eutectic. Show that the amount of A,B, and C in the assemblage of liquid, AC and BC just before final crystallization is the same as that in the starting liquid (65C,20A,15B). Show that the amount of A,B and C in the final crystallized mixture of A,AC and BC is the same as that in the starting liquid (65C, 20A, 15B). How much A is precipitated during final crystallization at the invariant point? i) Trace the crystallization path of a liquid containing 70%C,5%A and 25\% B. Be sure to describe ALL major events which occur as the temperature is lowered below the invariant point. Make quantitative calculations at temperatures where C is in equilibrium with liquid, where C and BC are in equilibrium with Hquld, where only BC is in equilibrium with liquid, where BC and A are in equilibrium with liquid and just before and just after final crystallization at the invariant point. How much B is precipitated during reaction at the invariant point? j) Draw the vertical section for the Join AC-BC. Assume reasonable temperature relationships for the system and label each area of the diagram for phases present. Use a quantitative scale for the horizontal composition axis. k) Draw the vertical section between C-c. Assume reasonable temperature relationships in the system and label each area of the diagram for phases present. Make sure the horizontal composition axis is reasonably quan titative between C and c
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


