Question: could you please explain how you reached the answer also as i am unsure how to. 5. Consider the following gas phase reaction @ 298K:
could you please explain how you reached the answer also as i am unsure how to. 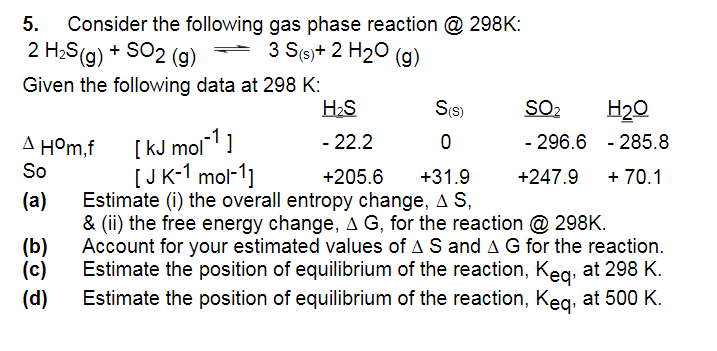
5. Consider the following gas phase reaction @ 298K: 2H2S(g)+SO2(g)3S(s)+2H2O(g) Given the following data at 298K : HOm,fSo[kJmol1][JK1mol1]22.2+205.60+31.9296.6+247.9285.8+70.1 (a) Estimate (i) the overall entropy change, S, \& (ii) the free energy change, G, for the reaction @ 298K. (b) Account for your estimated values of S and G for the reaction. (c) Estimate the position of equilibrium of the reaction, Keq, at 298K. (d) Estimate the position of equilibrium of the reaction, Keq, at 500K
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


