Question: Could you please hand write some help? Thank you for your help:-) 4. (12 pts) Phosgene, COCl2, is a colorless gas with a mildly sweet
Could you please hand write some help? Thank you for your help:-)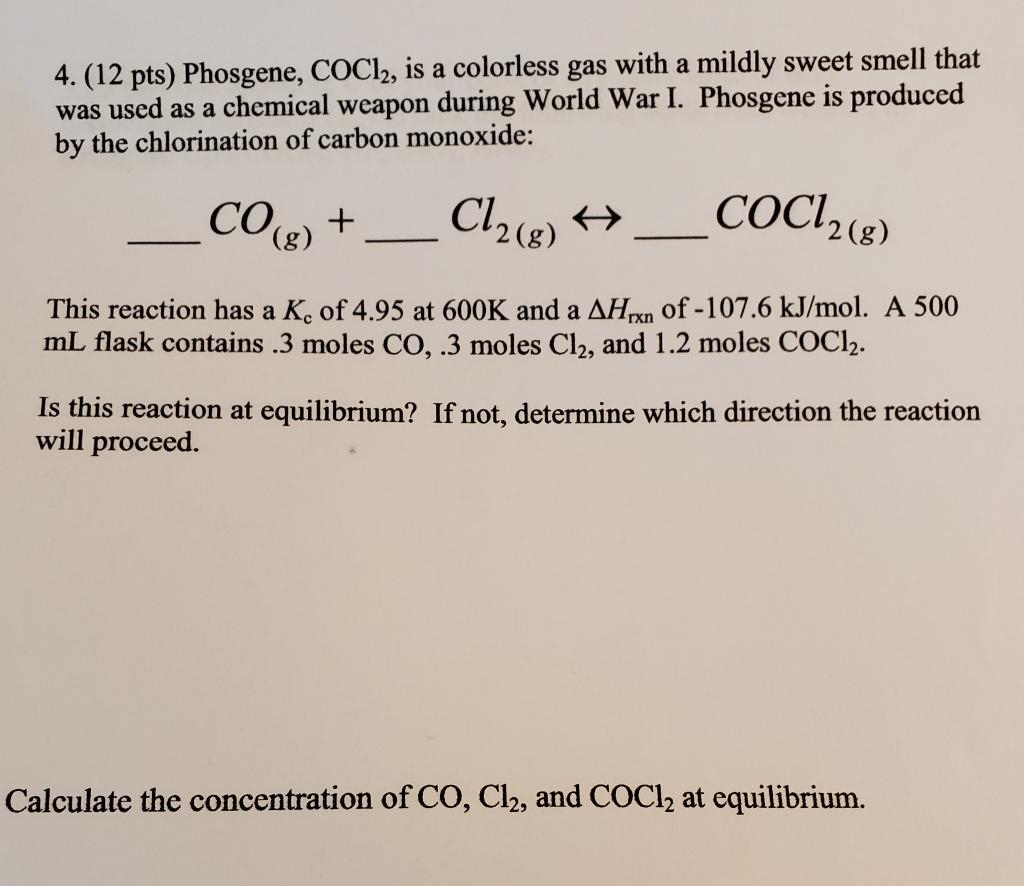
4. (12 pts) Phosgene, COCl2, is a colorless gas with a mildly sweet smell that was used as a chemical weapon during World War I. Phosgene is produced by the chlorination of carbon monoxide: CO(g) + Cl2(g) + __COC12(8) ) 8 - This reaction has a K. of 4.95 at 600K and a AHixn of -107.6 kJ/mol. A 500 mL flask contains .3 moles CO,.3 moles Cl2, and 1.2 moles COCl2. Is this reaction at equilibrium? If not, determine which direction the reaction will proceed. Calculate the concentration of CO, Cl2, and COCl2 at equilibrium
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


