Question: Could you please help in this? I understand there are two equivalence points. But why In the marking , it is mentioned that the first
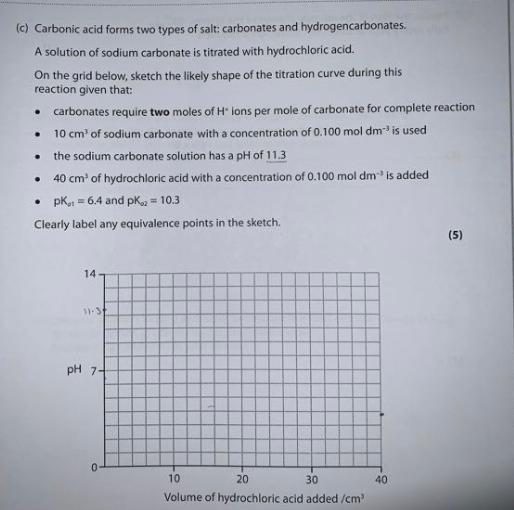
Could you please help in this? I understand there are two equivalence points.
But why In the marking , it is mentioned that the first vertical section must be around 10 cm^3 and the second vertical section is around 20 cm^3?
How to solve such questions!
(c) Carbonic acid forms two types of salt: carbonates and hydrogencarbonates. A solution of sodium carbonate is titrated with hydrochloric acid. On the grid below, sketch the likely shape of the titration curve during this reaction given that: carbonates require two moles of Hions per mole of carbonate for complete reaction 10 cm of sodium carbonate with a concentration of 0.100 mol dm- is used the sodium carbonate solution has a pH of 11.3 40 cm of hydrochloric acid with a concentration of 0.100 mol dmis added PK 64 and pk = 10.3 Clearly label any equivalence points in the sketch (5) . . 14 1.57 pH 7-4 0 40 10 20 30 Volume of hydrochloric acid added/cm
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


