Question: could you please help me work out these problem step by step for my pre lab. thanks 3. The total number of hydrogen atoms in
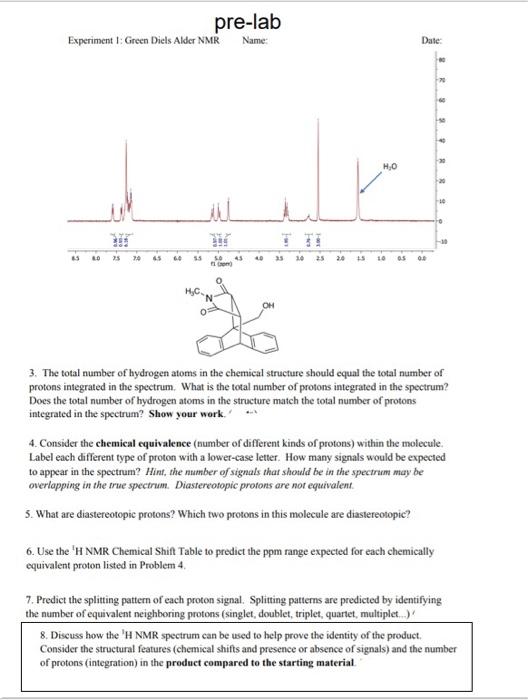
3. The total number of hydrogen atoms in the chemical structure should equal the total number of protons integrated in the spectrum. What is the total number of protons integrated in the spectrum? Does the total number of hydrogen atoms in the stracture match the total number of protons integrated in the spectrum? Show your work. 4. Consider the chemical equivalence (number of different kinds of protons) within the molecule. Label each different type of proton with a lower-case letter. How many signals would be expected to appear in the spectrum? Hint, the number of signals that should be in the spectram may be overlapping in the true spectrum. Diastereotopic protons are not equivalent. 5. What are diastereotopic protons? Which two protons in this molecule are diastereotopic? 6. Use the 1 H NMR Chemical Shift Table to predict the ppm range expected for each chemically equivalent proton listed in Problent 4. 7. Predict the splitting pattern of each proton signal. Splitting patterns are predicted by identifying the number of equivalent neighboring protons (singlet, doublet, triplet, quartet, multiplet..) 8. Discuss how the 'H NMR spectrum can be used to help prove the identity of the product. Consider the structural features (chemical shifts and presence or absence of signals) and the number of protons (integration) in the product compared to the starting material. 3. The total number of hydrogen atoms in the chemical structure should equal the total number of protons integrated in the spectrum. What is the total number of protons integrated in the spectrum? Does the total number of hydrogen atoms in the stracture match the total number of protons integrated in the spectrum? Show your work. 4. Consider the chemical equivalence (number of different kinds of protons) within the molecule. Label each different type of proton with a lower-case letter. How many signals would be expected to appear in the spectrum? Hint, the number of signals that should be in the spectram may be overlapping in the true spectrum. Diastereotopic protons are not equivalent. 5. What are diastereotopic protons? Which two protons in this molecule are diastereotopic? 6. Use the 1 H NMR Chemical Shift Table to predict the ppm range expected for each chemically equivalent proton listed in Problent 4. 7. Predict the splitting pattern of each proton signal. Splitting patterns are predicted by identifying the number of equivalent neighboring protons (singlet, doublet, triplet, quartet, multiplet..) 8. Discuss how the 'H NMR spectrum can be used to help prove the identity of the product. Consider the structural features (chemical shifts and presence or absence of signals) and the number of protons (integration) in the product compared to the starting material
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


