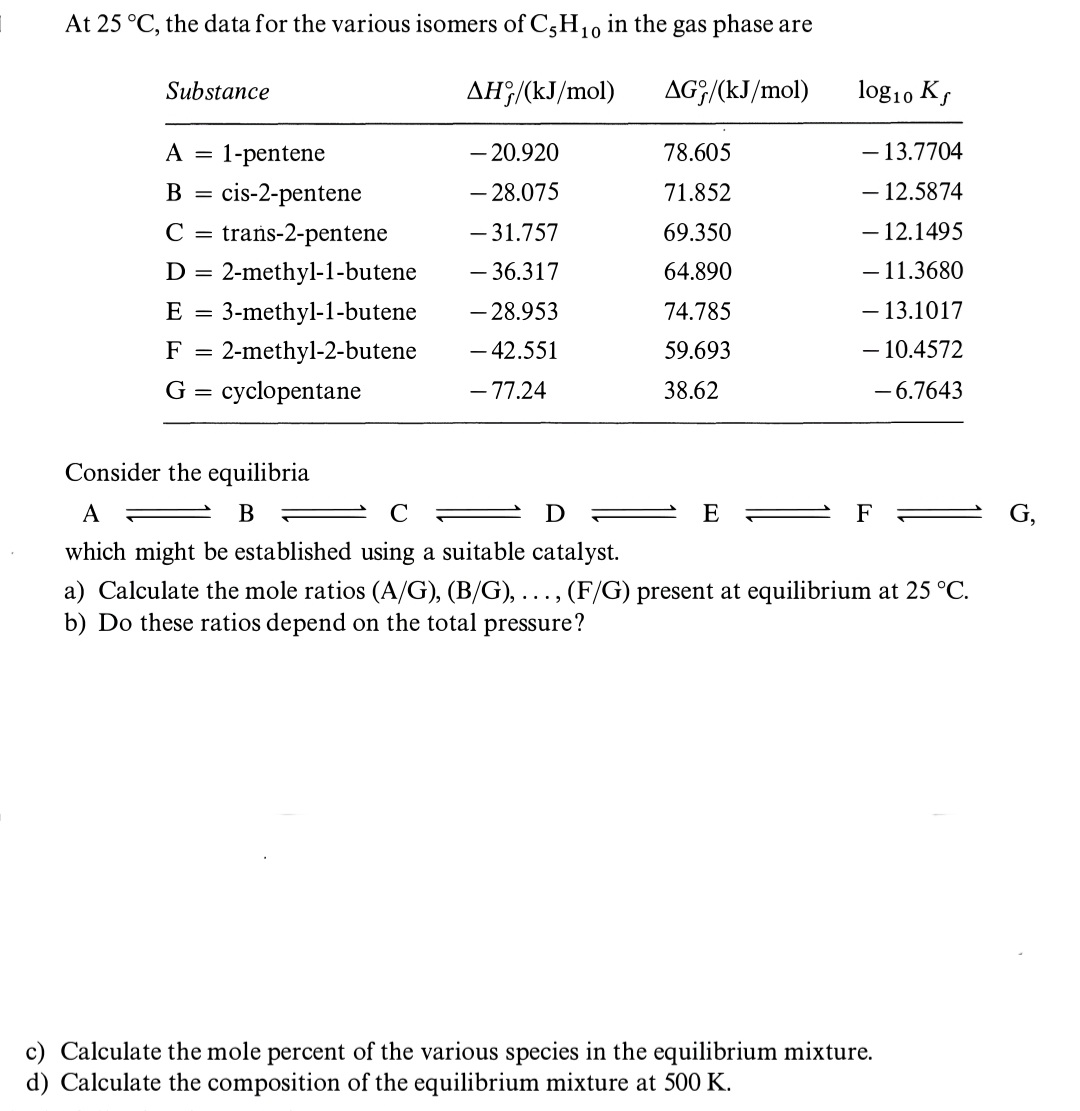
Question: Could you solve it step by step a), b), c) y d)?, please. Thank you so much in advance. At 25C, the data for the
 Could you solve it step by step a), b), c) y d)?, please. Thank you so much in advance.
Could you solve it step by step a), b), c) y d)?, please. Thank you so much in advance.
At 25C, the data for the various isomers of C5H10 in the gas phase are Consider the equilibria ABCDFF which might be established using a suitable catalyst. a) Calculate the mole ratios (A/G),(B/G),,(F/G) present at equilibrium at 25C. b) Do these ratios depend on the total pressure? c) Calculate the mole percent of the various species in the equilibrium mixture. d) Calculate the composition of the equilibrium mixture at 500K
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


