Question: Could you solve it step by step a), b) y c)?, please. Thank you so much in advance. For ozone at 25C,Gf=163.2kJ/mol a) At 25C,
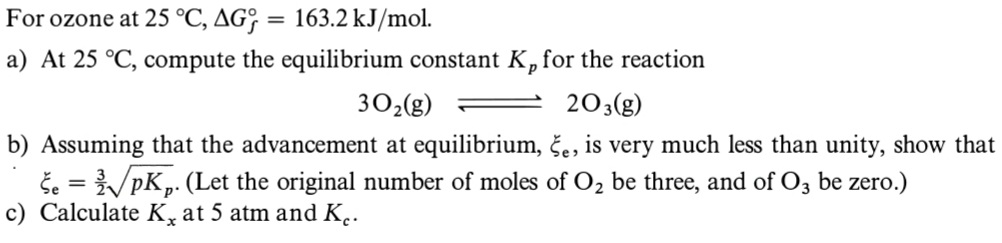
Could you solve it step by step a), b) y c)?, please. Thank you so much in advance.
For ozone at 25C,Gf=163.2kJ/mol a) At 25C, compute the equilibrium constant Kp for the reaction 3O2(g)2O3(g) b) Assuming that the advancement at equilibrium, e, is very much less than unity, show that e=23pKp. (Let the original number of moles of O2 be three, and of O3 be zero.) c) Calculate Kx at 5 atm and Kc
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


