Question: Could you solve it step by step?, please. Thanks in advance. Using the steady-state treatment, develop the rate expression for the following hypothetical mechanisms of
Could you solve it step by step?, please. Thanks in advance.
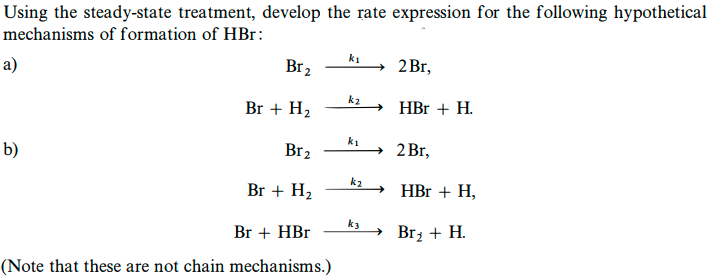
Using the steady-state treatment, develop the rate expression for the following hypothetical mechanisms of formation of HBr : a) b) Br2k12Br,Br+H2k2HBr+H.Br2k12Br,Br+H2k2HBr+H,Br+HBrk3Br2+H. (Note that these are not chain mechanisms.)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


