Question: Cp,m=a+bT where a and b are constants found by fitting experimental data. a) Find the value for H when 1.000mol of a substance is changed
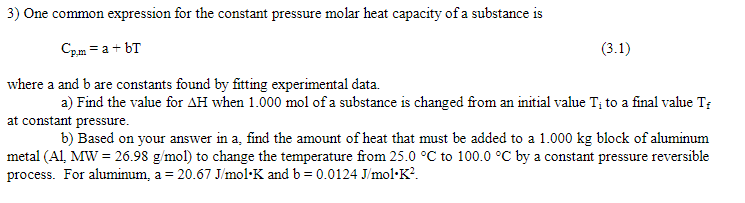
Cp,m=a+bT where a and b are constants found by fitting experimental data. a) Find the value for H when 1.000mol of a substance is changed from an initial value Ti to a final value Tf at constant pressure. b) Based on your answer in a, find the amount of heat that must be added to a 1.000kg block of aluminum metal (Al,MW=26.98g/mol) to change the temperature from 25.0C to 100.0C by a constant pressure reversible process. For aluminum, a=20.67J/molK and b=0.0124J/molK2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


