Question: Create Lewis dot structures for all major (nonequivalent) resonance forms. Include all formal charges on the appropriate atoms. For the central atoms (given) in the
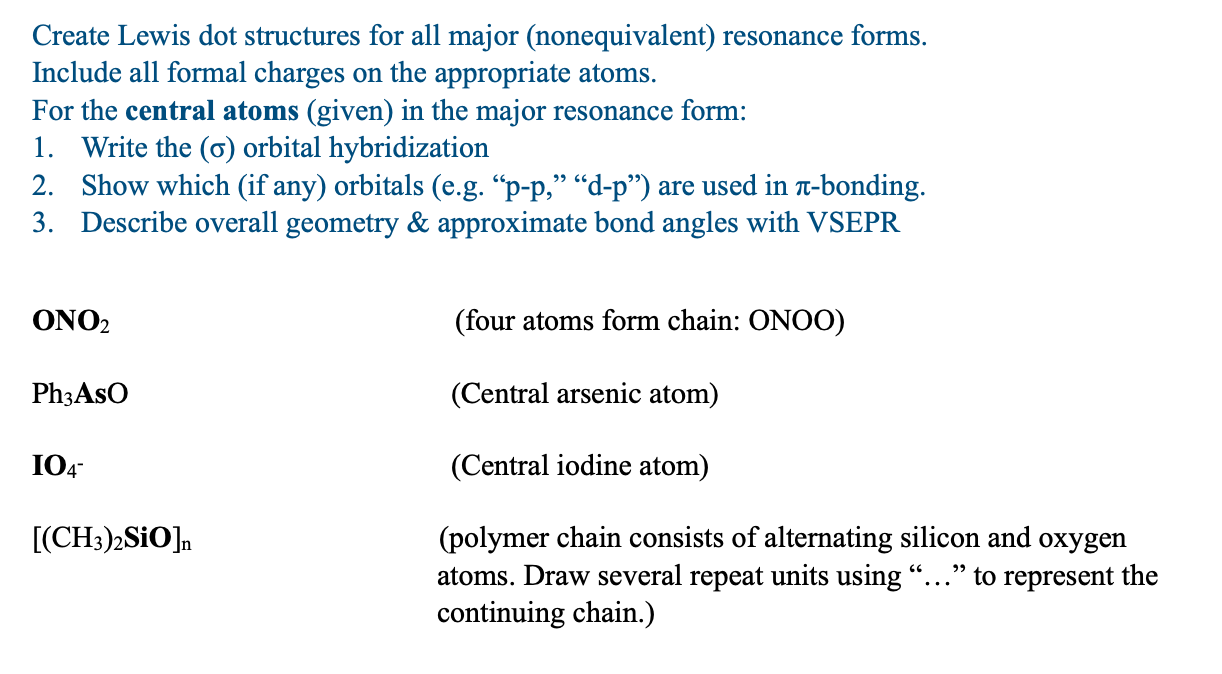
Create Lewis dot structures for all major (nonequivalent) resonance forms. Include all formal charges on the appropriate atoms. For the central atoms (given) in the major resonance form: 1. Write the () orbital hybridization 2. Show which (if any) orbitals (e.g. "p-p," "d-p") are used in -bonding. 3. Describe overall geometry \& approximate bond angles with VSEPR
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


