Question: Current Attempt in Progress A batch reactor is charged with 10.10kmol of sulfur dioxide and 5.80kmol cxygen. which react reversibly to form sulfur trioxide. The
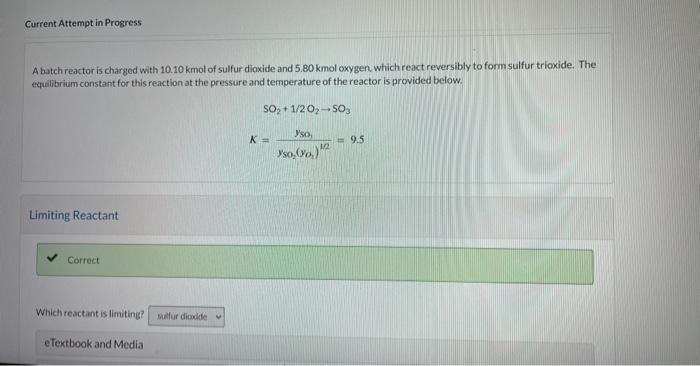
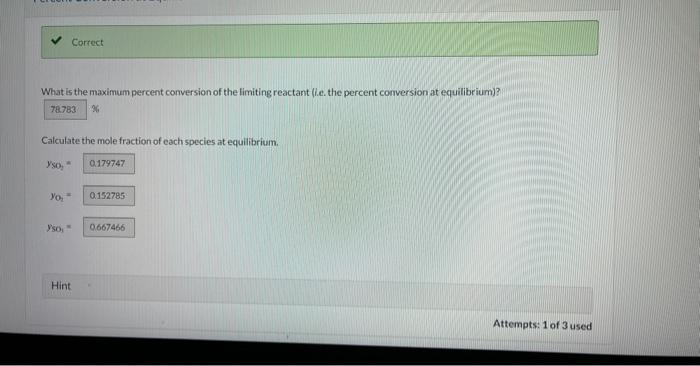
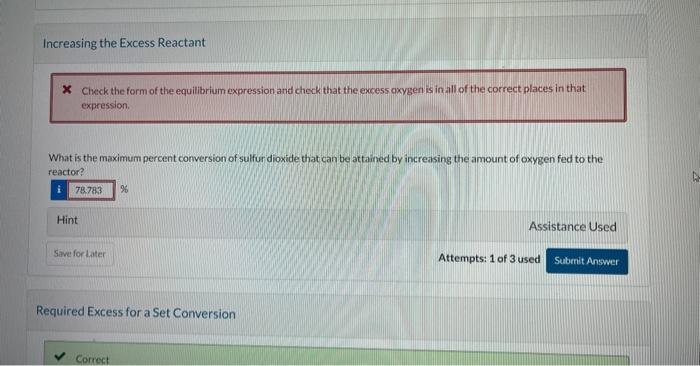
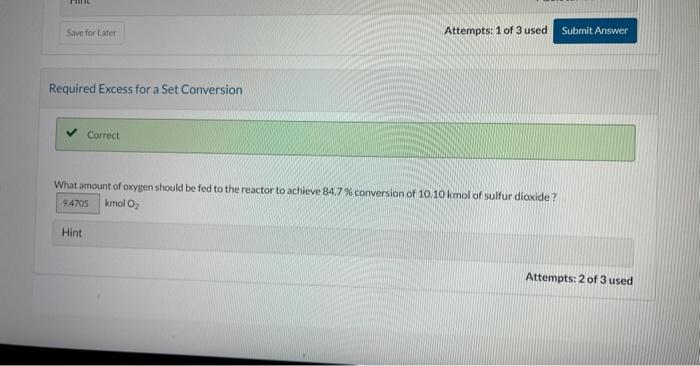
Current Attempt in Progress A batch reactor is charged with 10.10kmol of sulfur dioxide and 5.80kmol cxygen. which react reversibly to form sulfur trioxide. The equilibrium constant for this reaction at the pressure and temperature of the reactor is provided below. SO2+1/2O2SO3K=ySO2(yO2)1/2ySO=9.5 What is the maximum percent conversion of the limiting reactant (i.e. the percent conversion at equilibrium)? Qh. Calculate the mole fraction of each species at equilibrium. ySO2= yO2= ysosin= Increasing the Excess Reactant X Check the form of the equilibrium expression and chock that the excess oxygen is in all of the correct places in that expression. What is the maximum percent conversion of sulfur dioxide that can be attained by increasing the amount of oxygen fed to the reactor? Hint Assistance Used Attempts: 1 of 3 used Required Excess for a Set Conversion Required Excess for a Set Conversion What amount of oxygen should be fed to the reactor to achieve 84.7% conversion of 10.10kmol of sulfur dioxide? kmol2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


