Question: View Policies Current Attempt in Progress A batch reactor is charged with 13.10 kmol of sulfur dioxide and 6.80 kmol oxygen, which react reversibly to
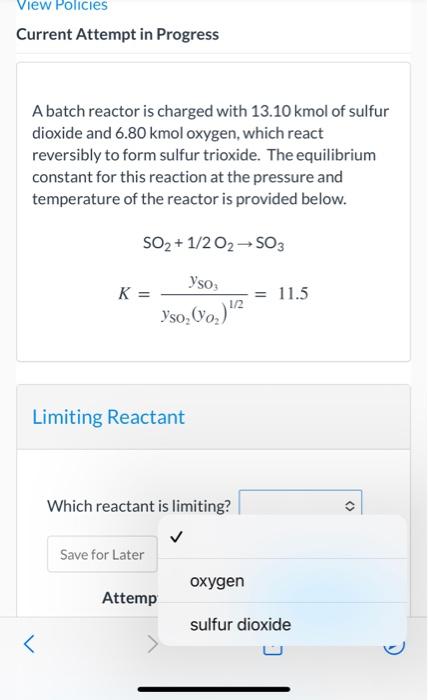
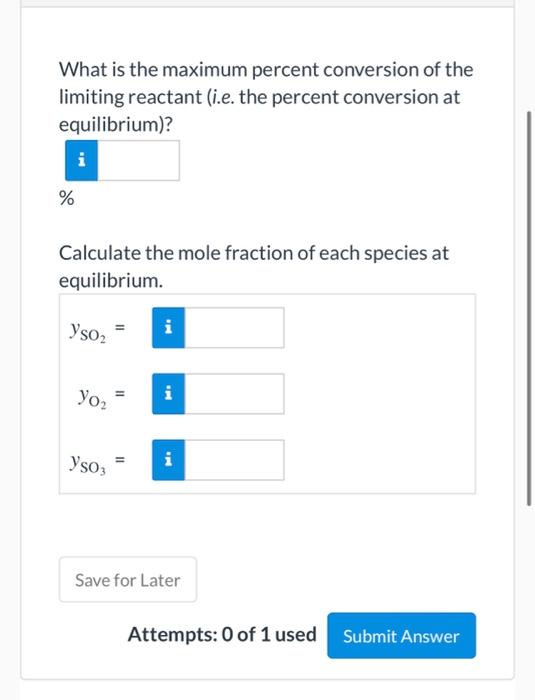
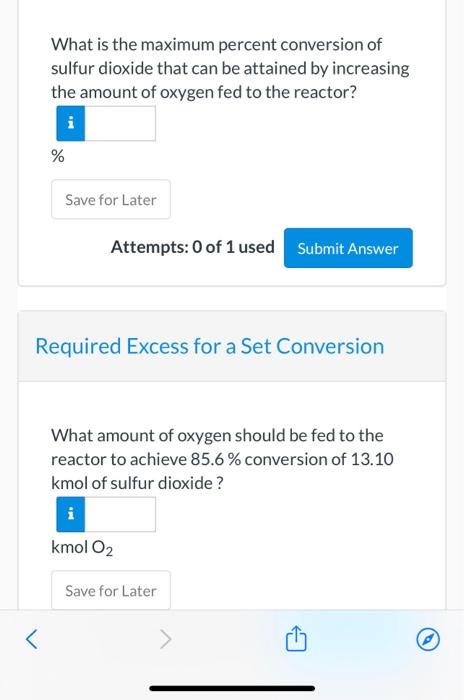
View Policies Current Attempt in Progress A batch reactor is charged with 13.10 kmol of sulfur dioxide and 6.80 kmol oxygen, which react reversibly to form sulfur trioxide. The equilibrium constant for this reaction at the pressure and temperature of the reactor is provided below. SO2+1/202-502 K= Yso = 11.5 Yso, (o,)'2 Limiting Reactant Which reactant is limiting? > Save for Later oxygen Attemp sulfur dioxide
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


