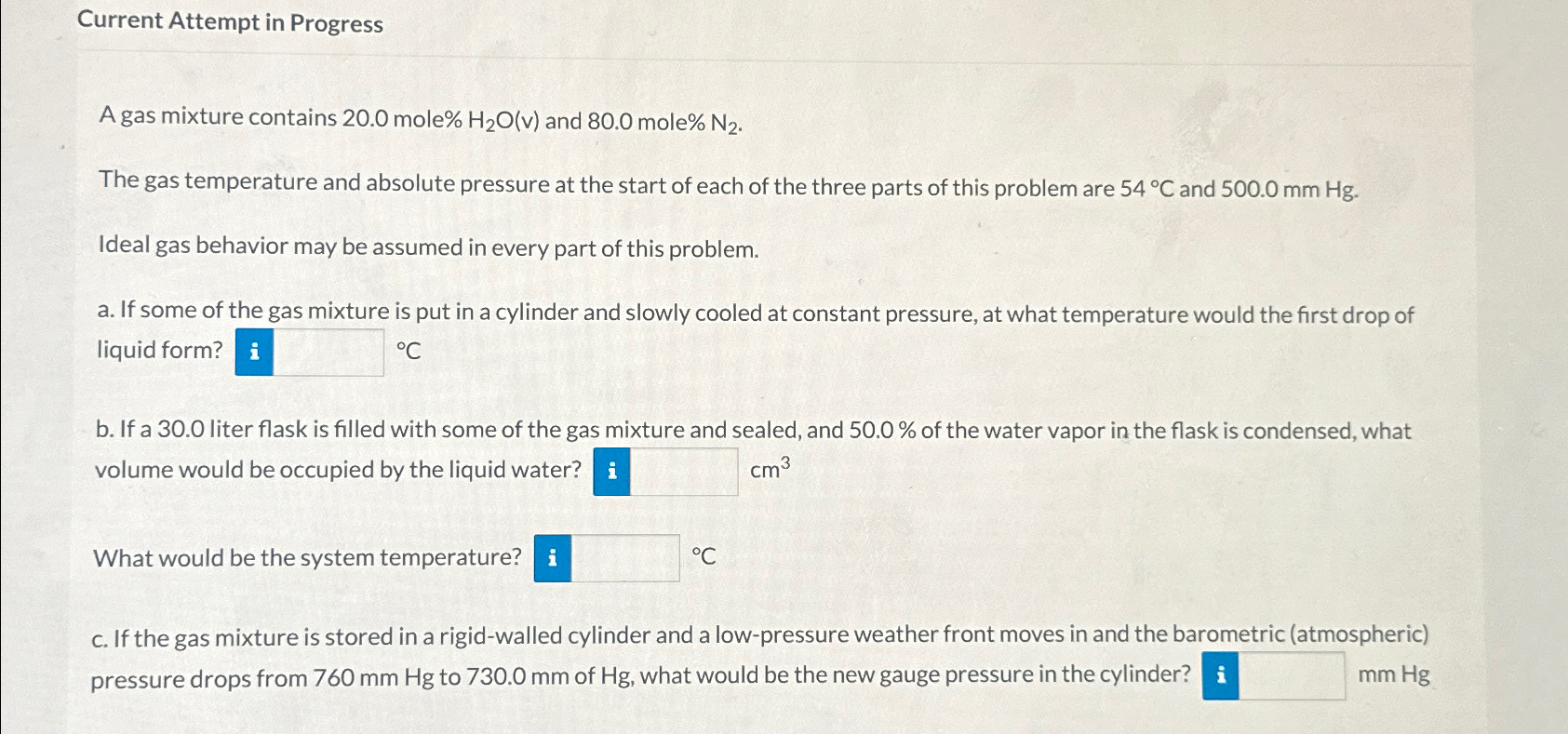
Question: Current Attempt in Progress A gas mixture contains 2 0 . 0 mole % H 2 O ( v ) and 8 0 . 0
Current Attempt in Progress
A gas mixture contains mole and mole
The gas temperature and absolute pressure at the start of each of the three parts of this problem are and Ideal gas behavior may be assumed in every part of this problem.
a If some of the gas mixture is put in a cylinder and slowly cooled at constant pressure, at what temperature would the first drop of liquid form?
b If a liter flask is filled with some of the gas mixture and sealed, and of the water vapor in the flask is condensed, what volume would be occupied by the liquid water?
What would be the system temperature?
c If the gas mixture is stored in a rigidwalled cylinder and a lowpressure weather front moves in and the barometric atmospheric pressure drops from to of what would be the new gauge pressure in the cylinder?
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


