Question: Current Attempt in Progress A rigid, insulated vessel is divided into two compartments connected by a valve. Initially, one compartment, occupying 1 . 0 f
Current Attempt in Progress
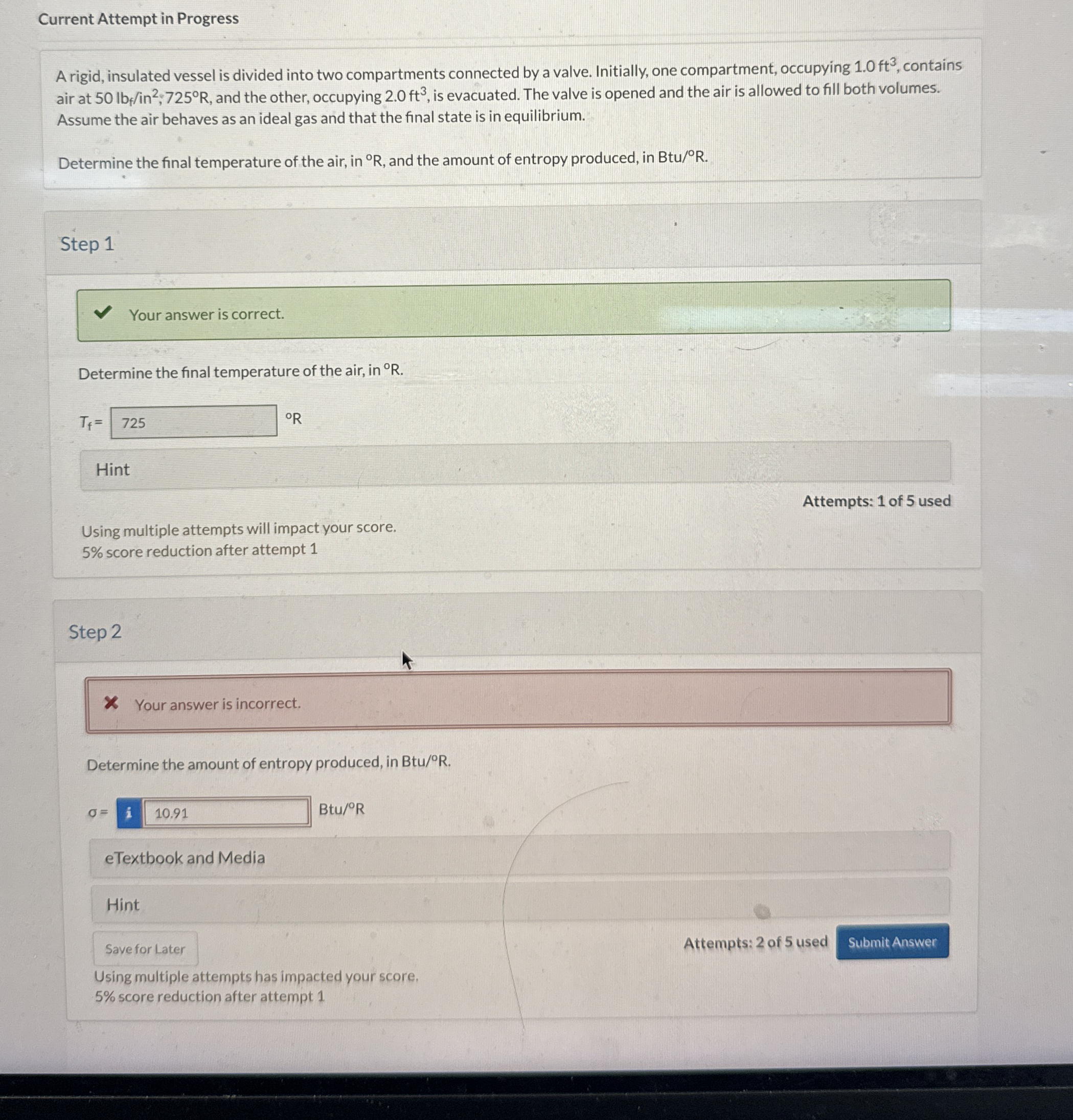
A rigid, insulated vessel is divided into two compartments connected by a valve. Initially, one compartment, occupying contains air at ; and the other, occupying is evacuated. The valve is opened and the air is allowed to fill both volumes. Assume the air behaves as an ideal gas and that the final state is in equilibrium.
Determine the final temperature of the air, in and the amount of entropy produced, in
Step
Your answer is correct.
Determine the final temperature of the air, in
Hint
Attempts: of used
Using multiple attempts will impact your score.
score reduction after attempt
Step
Your answer is incorrect.
Determine the amount of entropy produced, in
eTextbook and Media
Hint
Attempts: of used
Using multiple attempts has impacted your score.
score reduction after attempt
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


