Question: Current Attempt in Progress Examine the enthalpy diagram below and correctly place the enthalpy changes for a one-step conversion of germanium, Ge(5), into GeO2(s), the
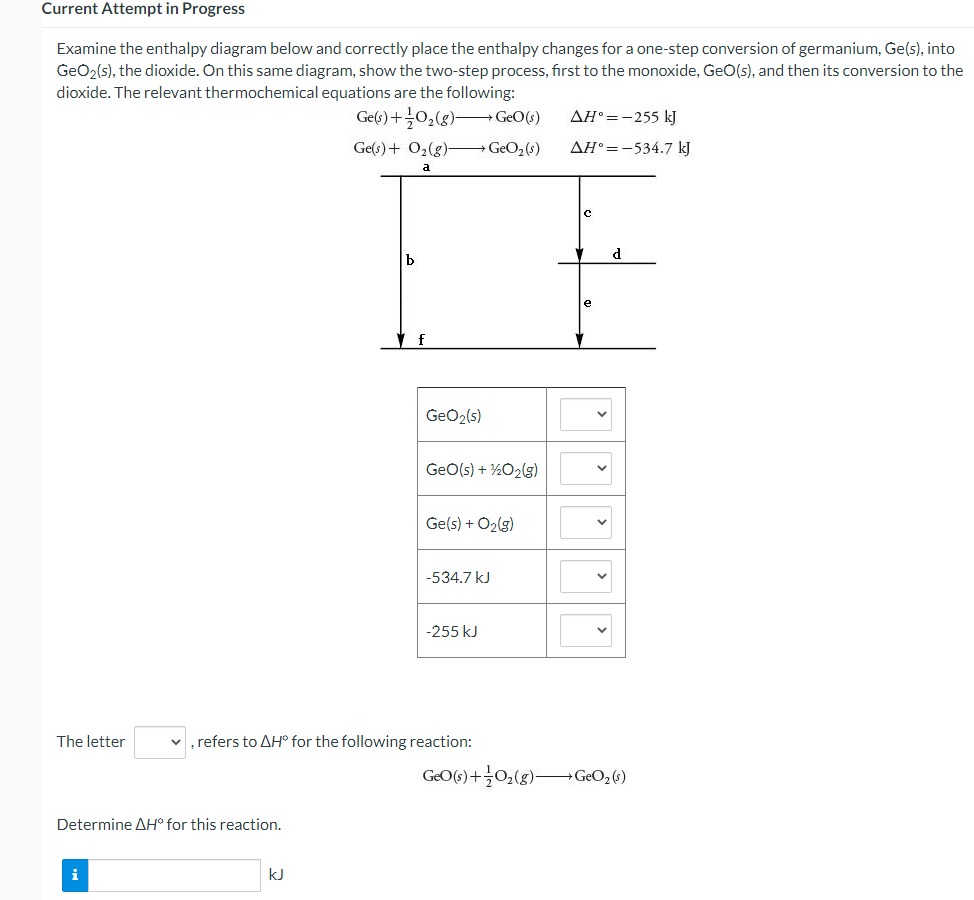
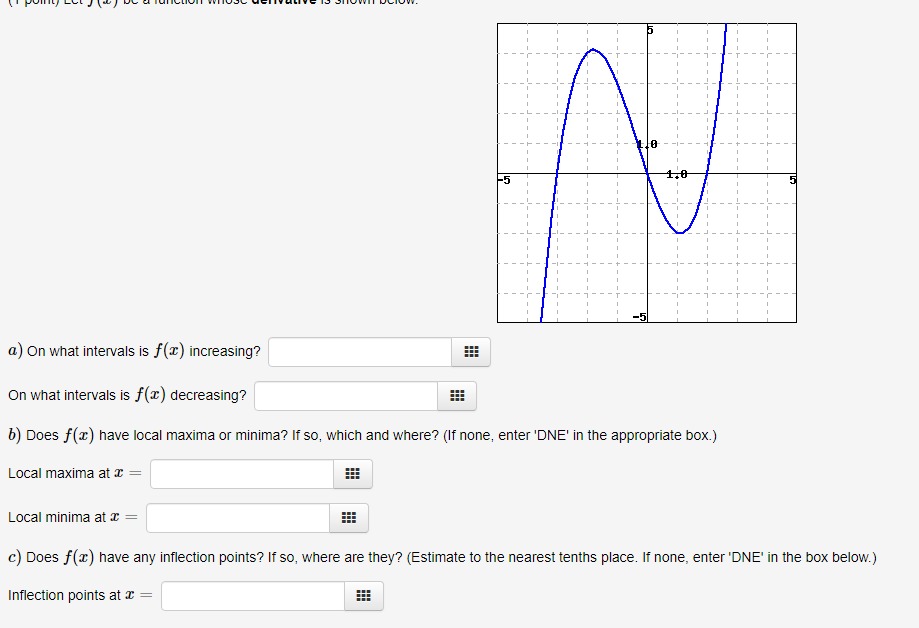

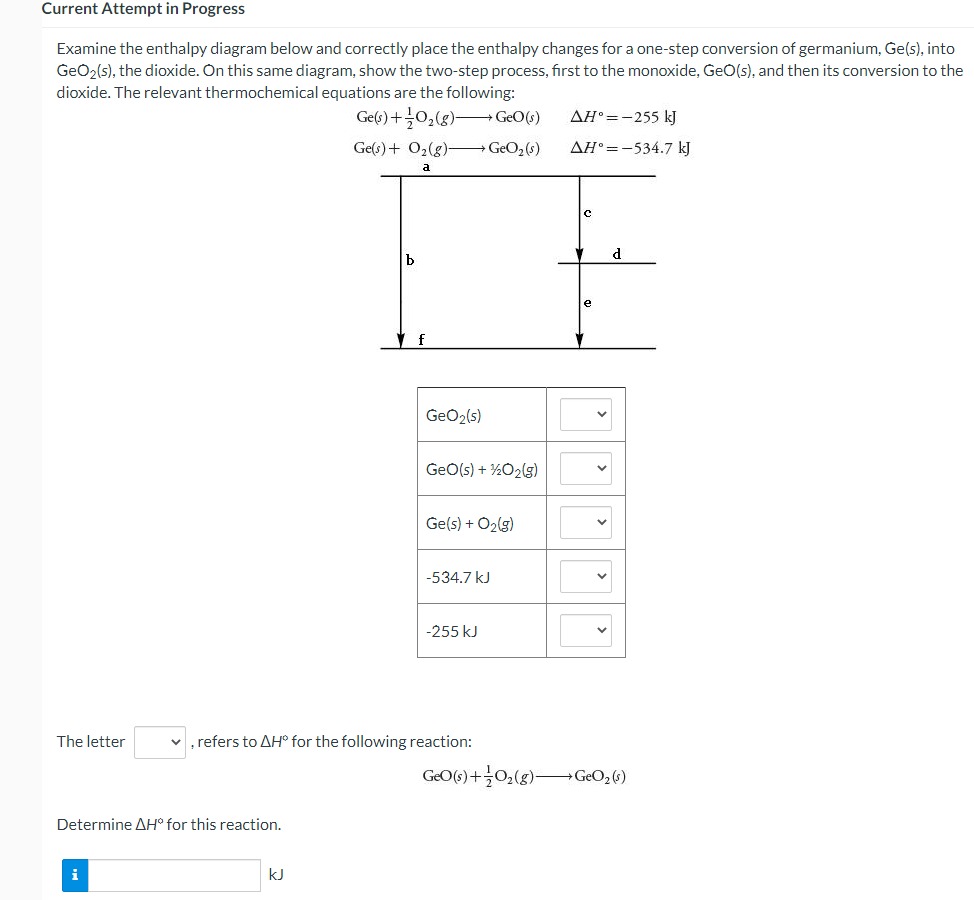
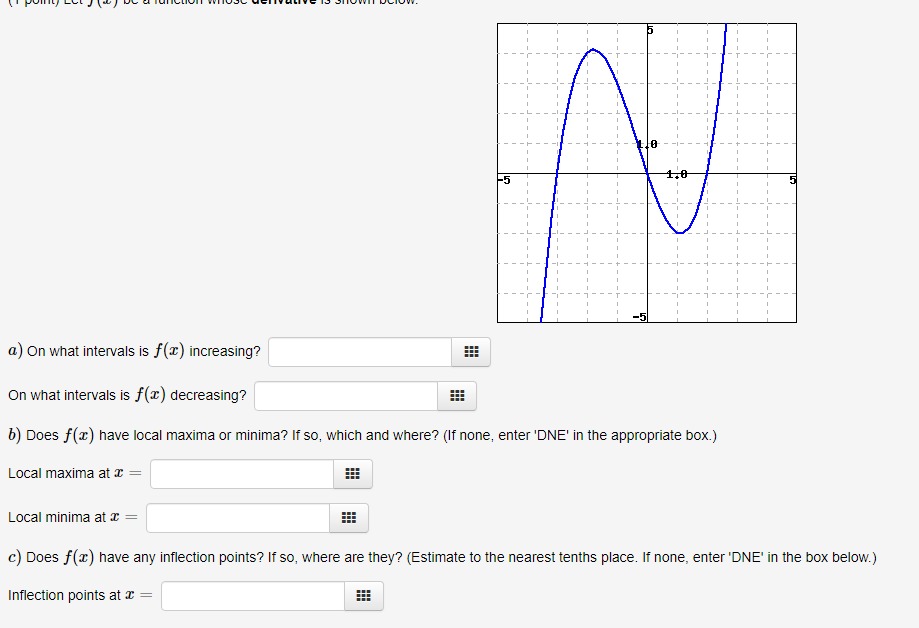

Current Attempt in Progress Examine the enthalpy diagram below and correctly place the enthalpy changes for a one-step conversion of germanium, Ge(5), into GeO2(s), the dioxide. On this same diagram, show the two-step process, first to the monoxide, GeO(s), and then its conversion to the dioxide. The relevant thermochemical equations are the following: Ge(s) + 702(8) + GeO(s) AH =-255 kJ Ge(s) + 02(g)- GeO, (s) AH.=-534.7 kJ a b d f GeO2(5) GeO(s) + 1202(g) Ge(s) + 02(5) -534.7 kJ -255 kJ The letter v, refers to AH for the following reaction: GEO(s) + 702(g)- GeO2 (5) Determine AH" for this reaction. i KJ1/0 -5 1.0 5 a) On what intervals is f() increasing? On what intervals is f() decreasing? b) Does f(x) have local maxima or minima? If so, which and where? (If none, enter 'DNE' in the appropriate box.) Local maxima at ~ = Local minima at ~ = c) Does f() have any inflection points? If so, where are they? (Estimate to the nearest tenths place. If none, enter 'DNE' in the box below.) Inflection points at c =The amino acid glycine, C2HsNO2, is one of the compounds used by the body to make proteins. The equation for its combustion is 4C2H;NO2(s) + 902(g) - 8CO2(g) +10H20(1) +2N2(5) For each mole of glycine that burns, 973.49 kJ of heat is liberated. Use this information, plus values of AH, for the products of combustion, to calculate AH," for glycine. i ! KJ/mol
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


