Question: Cu(s) + 2Ag+ (aq)Cu+ (aq) + 2Ag(s) Express the equilibrium constant to two significant digits. K= IVD Submit K- My Answers Give Up Part
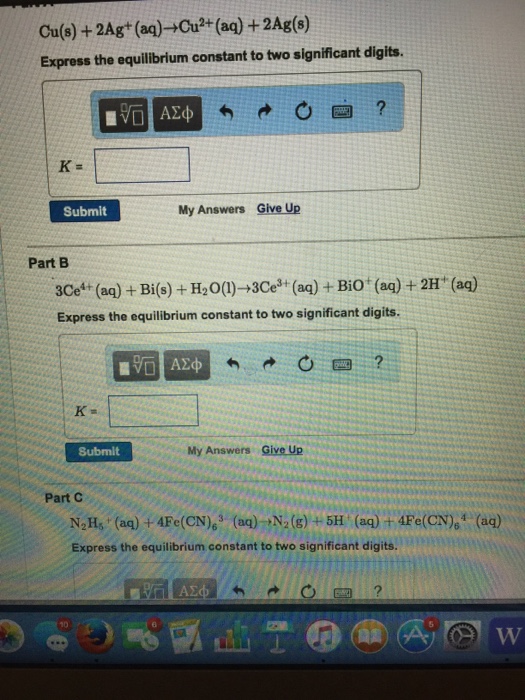
Cu(s) + 2Ag+ (aq)Cu+ (aq) + 2Ag(s) Express the equilibrium constant to two significant digits. K= IVD Submit K- My Answers Give Up Part B 3Ce+ (aq) + Bi(s) + HO(1)-3Ce+ (aq) + BiO (aq) + 2H+ (aq) Express the equilibrium constant to two significant digits. 15. Submit My Answers Give Up ? gyilk 1977 Part C NHs (aq) + 4Fe(CN)63 (aq) N(g) +5H (aq) + 4Fe(CN), (aq) Express the equilibrium constant to two significant digits. W
Step by Step Solution
3.41 Rating (157 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


