Question: D Question 2 2 pts You will need to generate a set of graphs to answer this question. Concentration vs time data for the following
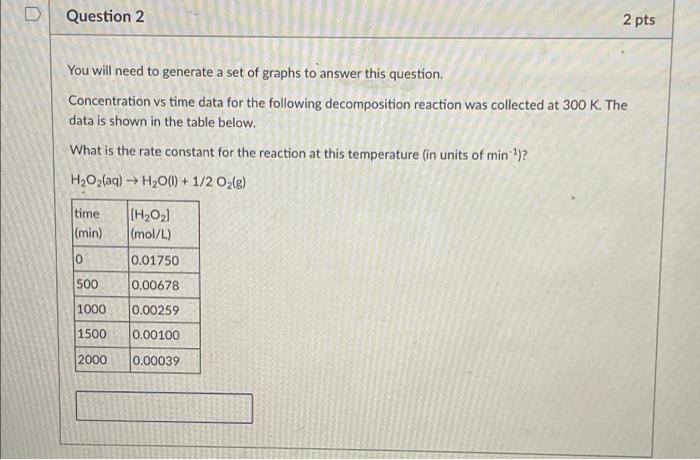
D Question 2 2 pts You will need to generate a set of graphs to answer this question. Concentration vs time data for the following decomposition reaction was collected at 300 K. The data is shown in the table below. What is the rate constant for the reaction at this temperature (in units of min)? H2O2(aq) H2O(l) + 1/2O2(e) time (min) [(HO21 (mol/L) 0 0.01750 500 0.00678 1000 0.00259 1500 0.00100 2000 0.00039
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


