Question: d) The following data is obtained from the adsorption experiment of nitrogen gas on 1.75g of minerals at 25 C. The saturated pressure is 76.0
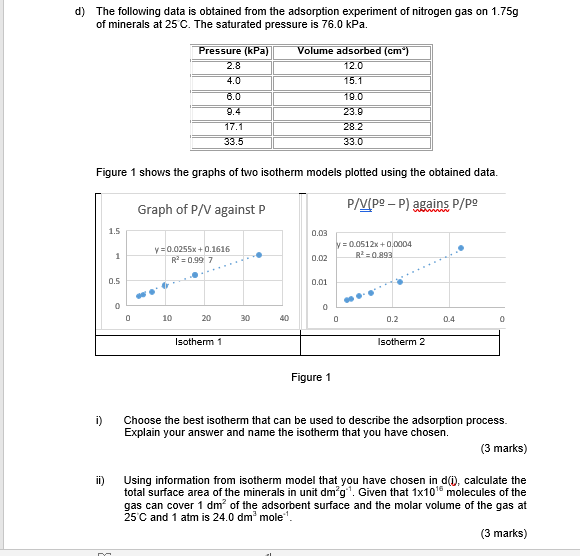
d) The following data is obtained from the adsorption experiment of nitrogen gas on 1.75g of minerals at 25 C. The saturated pressure is 76.0 kPa. Pressure (kPa) 2.8 4.0 6.0 9.4 17.1 33.5 Volume adsorbed (cm) 12.0 15.1 19.0 23.9 28.2 33.0 Figure 1 shows the graphs of two isotherm models plotted using the obtained data. Graph of P/V against P P/V(P-P) agains P/P 1.5 0.03 y = 0.0512x+0.0004 R2 = 0.893 y = 0.0255x+0.1616 R = 0.997 1 0.02 0.5 0.01 0 0 0 10 20 30 40 0 0.2 0.4 0 Isotherm 1 Isotherm 2 Figure 1 i) Choose the best isotherm that can be used to describe the adsorption process. Explain your answer and name the isotherm that you have chosen. (3 marks) Using information from isotherm model that you have chosen in do, calculate the total surface area of the minerals in unit dm'g". Given that 1x10' molecules of the gas can cover 1 dm? of the adsorbent surface and the molar volume of the gas at 25 C and 1 atm is 24.0 dm mole
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


