Question: DATA AND CALCULATIONS Kc expression Average of KC values Sample Calculations to be done on the next page. 1. Write the Ke expression for the
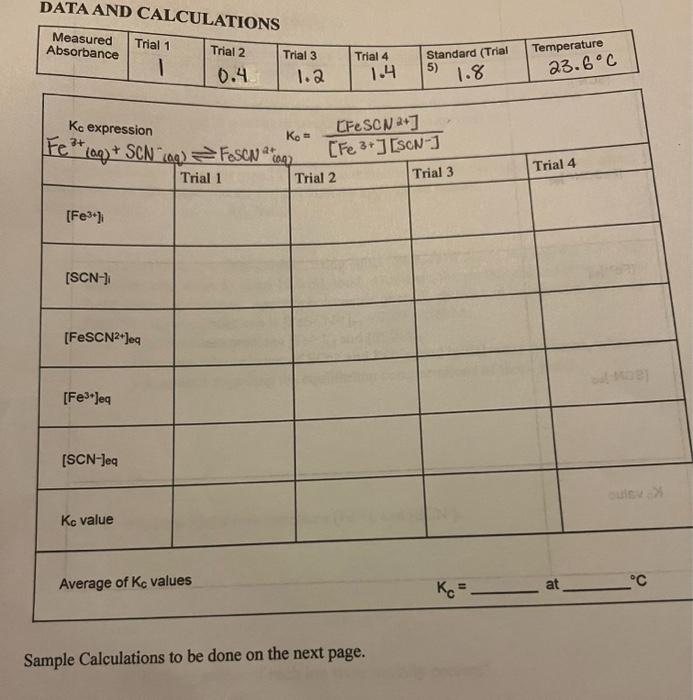



DATA AND CALCULATIONS Kc expression Average of KC values Sample Calculations to be done on the next page. 1. Write the Ke expression for the reaction in the Data and Calculation table. 2. Calculate the initial concentration of Fe3, based on the dilution that results from adding KSCN solution and water to the original 0.0020MFe(NO3) s solution. See Step 2 of the procedure for the volume of each substance used in Trials 1-4. Calculate [Fe3+] ising the equation: [Fe3+]i=x(0.0020M) This should be the same for all four test tubes. 3. Calculate the initial concentration of SCN, based on its dilution by Fe(NO3)3 and water: [SCN]i=x(0.0020M) In Test Tube 1, [SCN]i=(2mL/10mL)(.0020M)=,00040M. Caleulate this for the other three test tubes. 4. [FeSCN2]cq is calculated using the formula: [FeSCN2+]eq=x[FeSCN2+]sad where Aeq and Astd are the absorbance valves for the equilibrium and standard test tubes, respectively, and [FeSCN2+]std=(1/10)(0.0020)=0.00020M. Recall that [FeSCN+]std is assumed to be equal to [SCN]r. Calculate [FeSCN2]eq for each of the four trials. 5. [Fe3+]eq: Calculate the concentration of Fe3+ at equilibrium for Trials 14 using the equation: [Fe3+]eq=[Fe3+]i[FeSCN2]eq 6. [SCN]eq : Calculate the concentration of SCN at equilibrium for Trials 14 using the equation: [SCN]eq=[SCN]1[FeSCN+]cq 7. Calculate Kc for Trials 14. Be sure to show the Kc expression and the values substituted in for each of these calculations. 8. Using your four calculated Kc values, determine an average value for Kc. How constant were your Kc values? Ramette, R.W. J. Chem Ed. 1. Compare your value to this literature value. Calculate the percent error. Is your experimental value in good agreement? If not, offer an explanation. If your value differs by greater than 15%, use the literature value for the following calculations. Indicate here which value you are using (circle the appropriate value): Literature Value or My Value 2. A student prepared a fifth solution, using equal volumes of the SCN(aq) and Fe3+ (aq) solutions, plus water to equal 10.0mL total. However, the student did not remember to Write down the volumes. Spectrophotometric analysis shows that the concentration of FeSCN2+(aq) at equilibrium is [FeSCN2+]eq=7.29105M. a. Find the concentrations [Fe3+]eq and [SCN]eq. Hint: since the volumes of SCNand Fe3+ added initial were the same, what can you assume regarding the equilibrium concentrations of these ions? b. Find the initial concentrations [Fe3+]i and [SCN]i. c. How many moles of each ion were initially present? xpperiment 5 d. What initial volume of each solution was used
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


