Question: DATA Data is anything that you measured or observed. Record data directly info your notebook inchindirg the units. Use ink. When you make an emor,
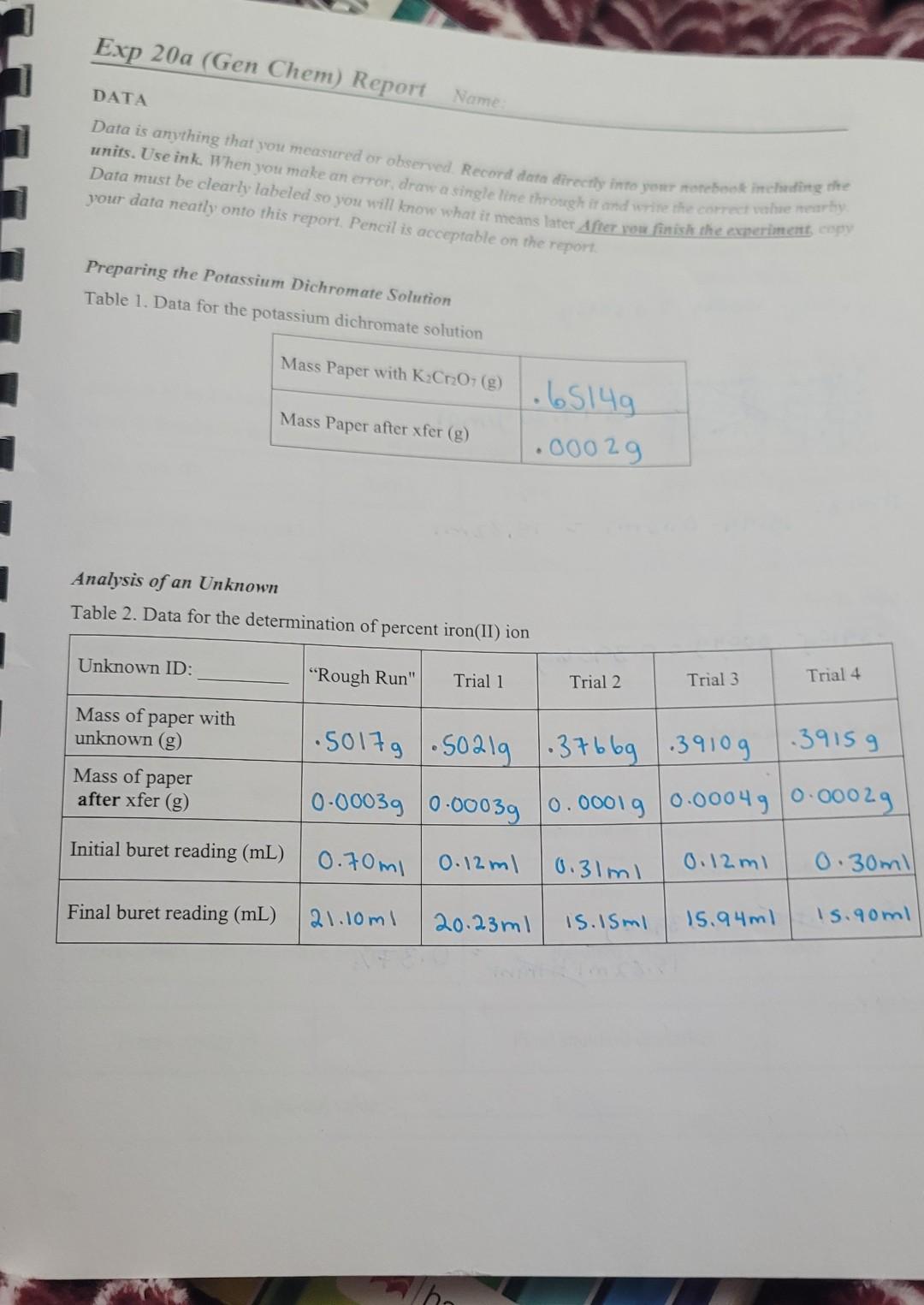

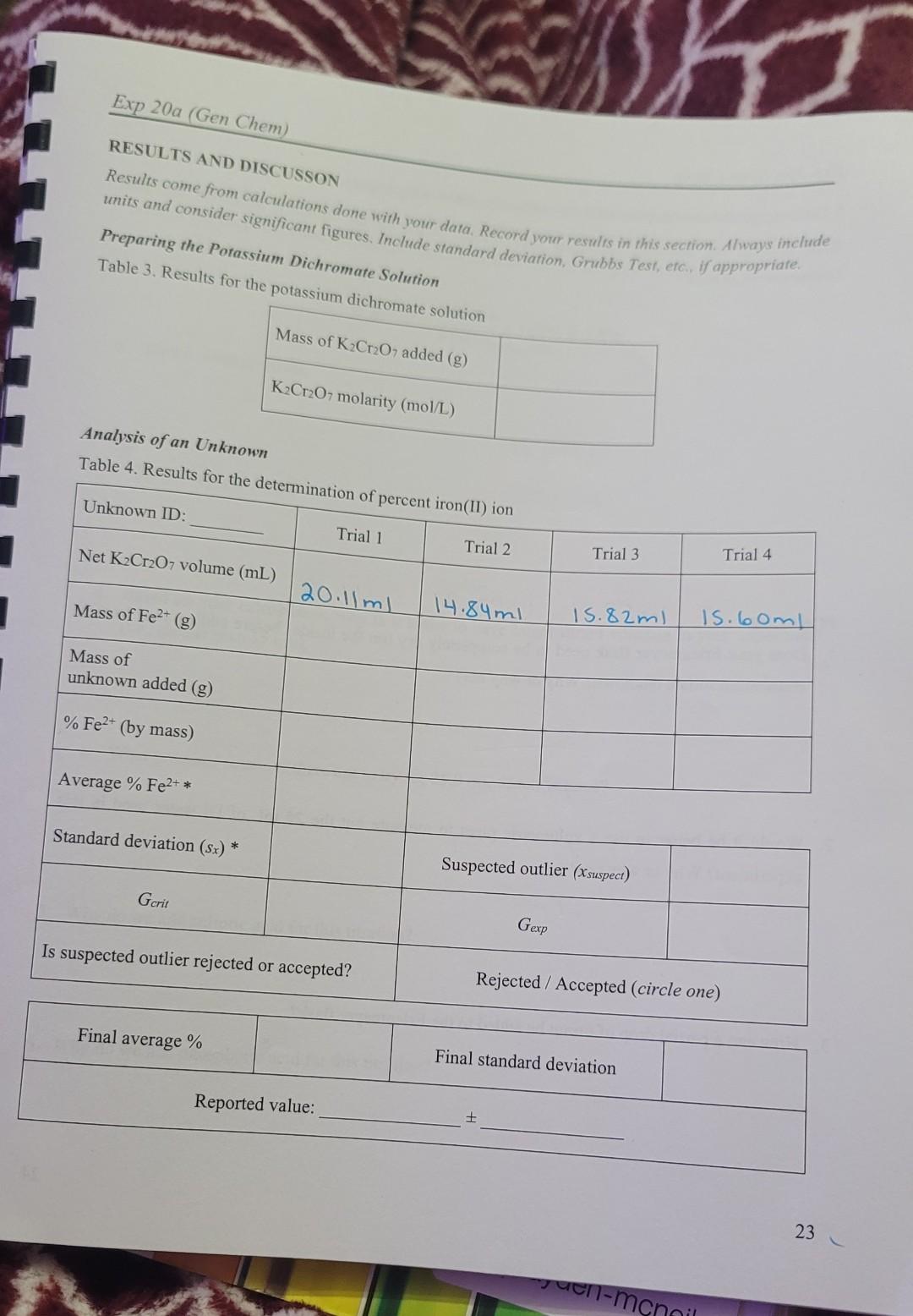
DATA Data is anything that you measured or observed. Record data directly info your notebook inchindirg the units. Use ink. When you make an emor, drave a single line through it and wrine the corred vahue meartiy? Data must be clearly labeled so you will know what it means later After wou finish the experinent copy your data neatly onto this report. Pencil is acceptable on the report. Preparing the Potassium Dichromate Solution Table 1. Data for the potassium Analysis of an Unknown Table 2. Data for the determination of percent iron(II) ion CALCULATIONS Show one example of each calculation, including umits. I have labeled the calcularions your should show for this experiment. Work out your calculations in your notebook first and then copy them neatly here. Preparing the Potassium Dichromate Solution Mass of K2Cr2O7 added: .6514g.0002g=0.6512g K2C2O7 molarity: Analysis of an Unknown Net volume of K2Cr2O7 used: Mass of Fe2+ : Mass of unknown added: %Fe2+ (by mass) in unknown: Grubbs Test: RESULTS AND DISCUSSON Results come from calculations done with your data. Record your results in this section. Always include, units and consider significant figures, Include standard deviation, Grubbs Test, etc., if appropriate. Preparing the Potassium Dichromate Solution Table 3. Results for the potacis Analysis of an Unkno Table 4. Results for tha , DATA Data is anything that you measured or observed. Record data directly info your notebook inchindirg the units. Use ink. When you make an emor, drave a single line through it and wrine the corred vahue meartiy? Data must be clearly labeled so you will know what it means later After wou finish the experinent copy your data neatly onto this report. Pencil is acceptable on the report. Preparing the Potassium Dichromate Solution Table 1. Data for the potassium Analysis of an Unknown Table 2. Data for the determination of percent iron(II) ion CALCULATIONS Show one example of each calculation, including umits. I have labeled the calcularions your should show for this experiment. Work out your calculations in your notebook first and then copy them neatly here. Preparing the Potassium Dichromate Solution Mass of K2Cr2O7 added: .6514g.0002g=0.6512g K2C2O7 molarity: Analysis of an Unknown Net volume of K2Cr2O7 used: Mass of Fe2+ : Mass of unknown added: %Fe2+ (by mass) in unknown: Grubbs Test: RESULTS AND DISCUSSON Results come from calculations done with your data. Record your results in this section. Always include, units and consider significant figures, Include standard deviation, Grubbs Test, etc., if appropriate. Preparing the Potassium Dichromate Solution Table 3. Results for the potacis Analysis of an Unkno Table 4. Results for tha
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


