Question: Data from Lab is included. Please help solve problems. Data Sheet 16 Total water hardness (Step 2) Average ppm CaCO3590 Temporary water hardness (Step 6)
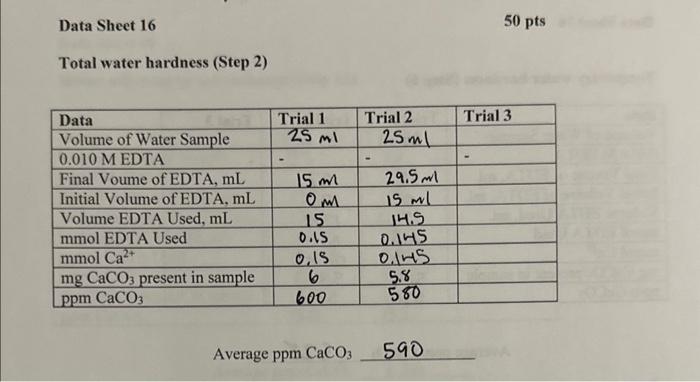
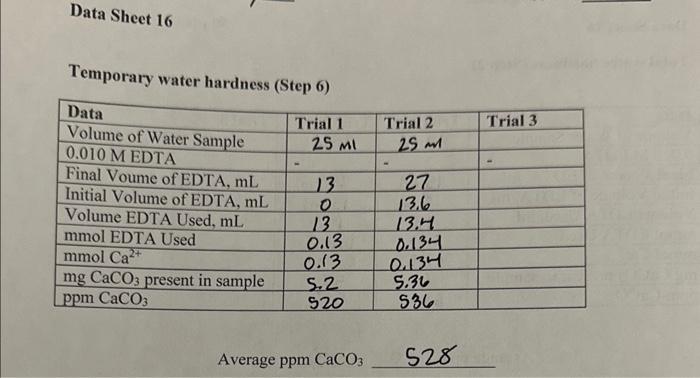
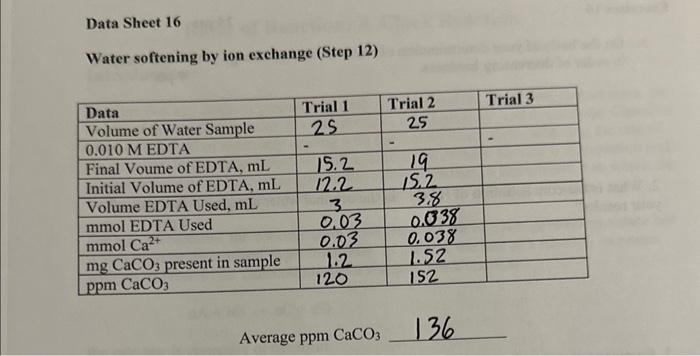

Data Sheet 16 Total water hardness (Step 2) Average ppm CaCO3590 Temporary water hardness (Step 6) Average ppm CaCO3 528 Water softening by ion exchange (Step 12) Average ppmCaCO3136 1. On the basis of your determination of water hardness, arrange your water samples in order of decreasing hardness. 9pts 2. What is the concentration of HCO3in the original water sample on the basis of your results? 6pts 3. Suppose you titrated a sample of distilled water with EDTA, approximately what volume of ETDA would be required? 5pts 4. Write a balanced chemical equation for the exchange reaction which occurred in Part 16; when the water sample was passed over the ion exchange column. 5pts Data Sheet 16 Total water hardness (Step 2) Average ppm CaCO3590 Temporary water hardness (Step 6) Average ppm CaCO3 528 Water softening by ion exchange (Step 12) Average ppmCaCO3136 1. On the basis of your determination of water hardness, arrange your water samples in order of decreasing hardness. 9pts 2. What is the concentration of HCO3in the original water sample on the basis of your results? 6pts 3. Suppose you titrated a sample of distilled water with EDTA, approximately what volume of ETDA would be required? 5pts 4. Write a balanced chemical equation for the exchange reaction which occurred in Part 16; when the water sample was passed over the ion exchange column. 5pts
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


