Question: Data in the table were collected at 540K for the following reaction: CO(g)+NO2(g)CO2(g)+NO(g) a Determine the reaction order with respect to each reactant. Order with
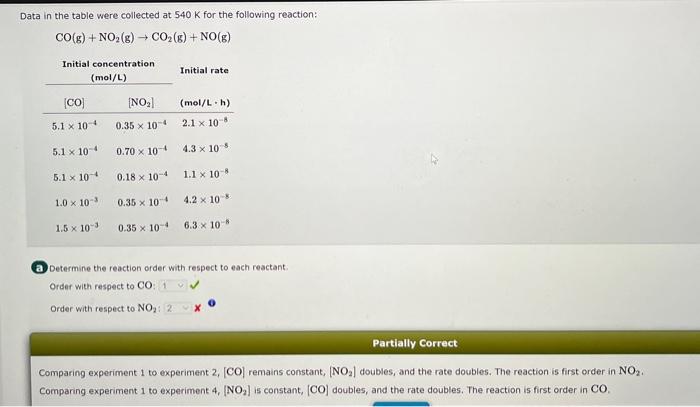
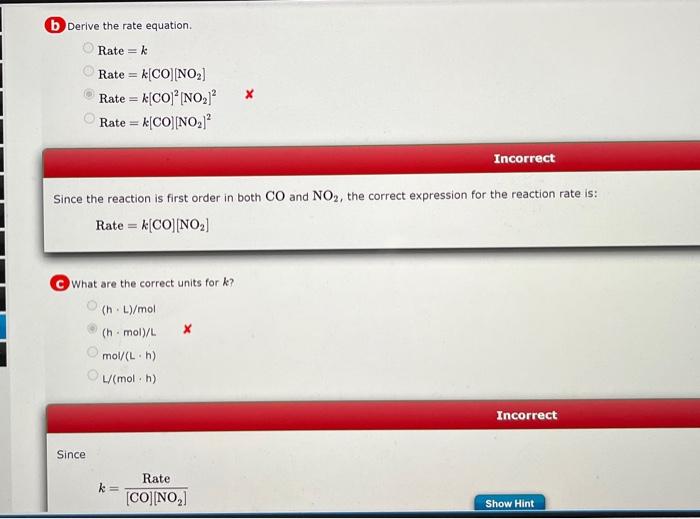

Data in the table were collected at 540K for the following reaction: CO(g)+NO2(g)CO2(g)+NO(g) a Determine the reaction order with respect to each reactant. Order with respect to CO Order with respect to NO2 i x0 Partially Correct Comparing experiment 1 to experiment 2,[CO] remains constant, [NO2] doubles, and the rate doubles. The reaction is first order in NO2. Comparing experiment 1 to experiment 4,[NO2] is constant, [CO] doubles, and the rate doubles. The reaction is first order in CO. (b) Derive the rate equation. Rate=kRate=k[CO][NO2]Rate=k[CO]2[NO2]2XRate=k[CO][NO2]2 Incorrect Since the reaction is first order in both CO and NO2, the correct expression for the reaction rate is: Rate=k[CO][NO2] C What are the correct units for k ? (hL)/mol(hmol)/Lmol/Lh)L(molh) Since k=[CO][NO2]Rate d Calculate the rate constant. Rateconstant=L/molh) From the rate equation, k=[CO][NO2]Rate=(5.1104mol/L)(0.35104mol/L)(2.1108mol/(Lh))=1.2L/(molh)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


