Question: Data is collected for the gas phase reaction 2 A + B + 3 C Products at 470 K. Use the rate law Rate =
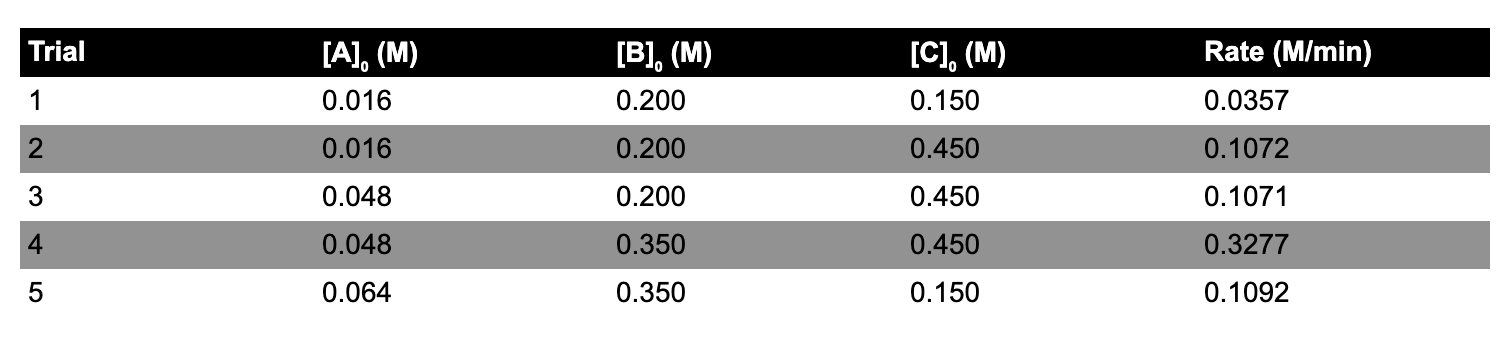 Data is collected for the gas phase reaction 2 A + B + 3 C Products at 470 K. Use the rate law Rate = k[B][C]. What is the value of the rate constant at 470 K for this reaction?
Data is collected for the gas phase reaction 2 A + B + 3 C Products at 470 K. Use the rate law Rate = k[B][C]. What is the value of the rate constant at 470 K for this reaction?
\begin{tabular}{lllll} \hline Trial & {[A0(M)} & {[B0(M)} & {[C0(M)} & Rate (M/min) \\ \hline 1 & 0.016 & 0.200 & 0.150 & 0.0357 \\ 2 & 0.016 & 0.200 & 0.450 & 0.1072 \\ 3 & 0.048 & 0.200 & 0.450 & 0.1071 \\ 4 & 0.048 & 0.350 & 0.450 & 0.3277 \\ 5 & 0.064 & 0.350 & 0.150 & 0.1092 \end{tabular}
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


