Question: Please provide the polymath script for the conversions given above The gas phase reaction A+BC follows an elementary rate law and occurs in a 1m3CSTR.



Please provide the polymath script for the conversions given above
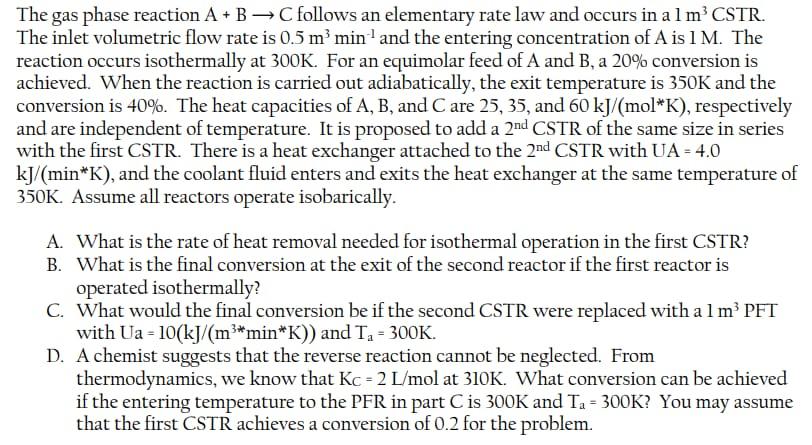
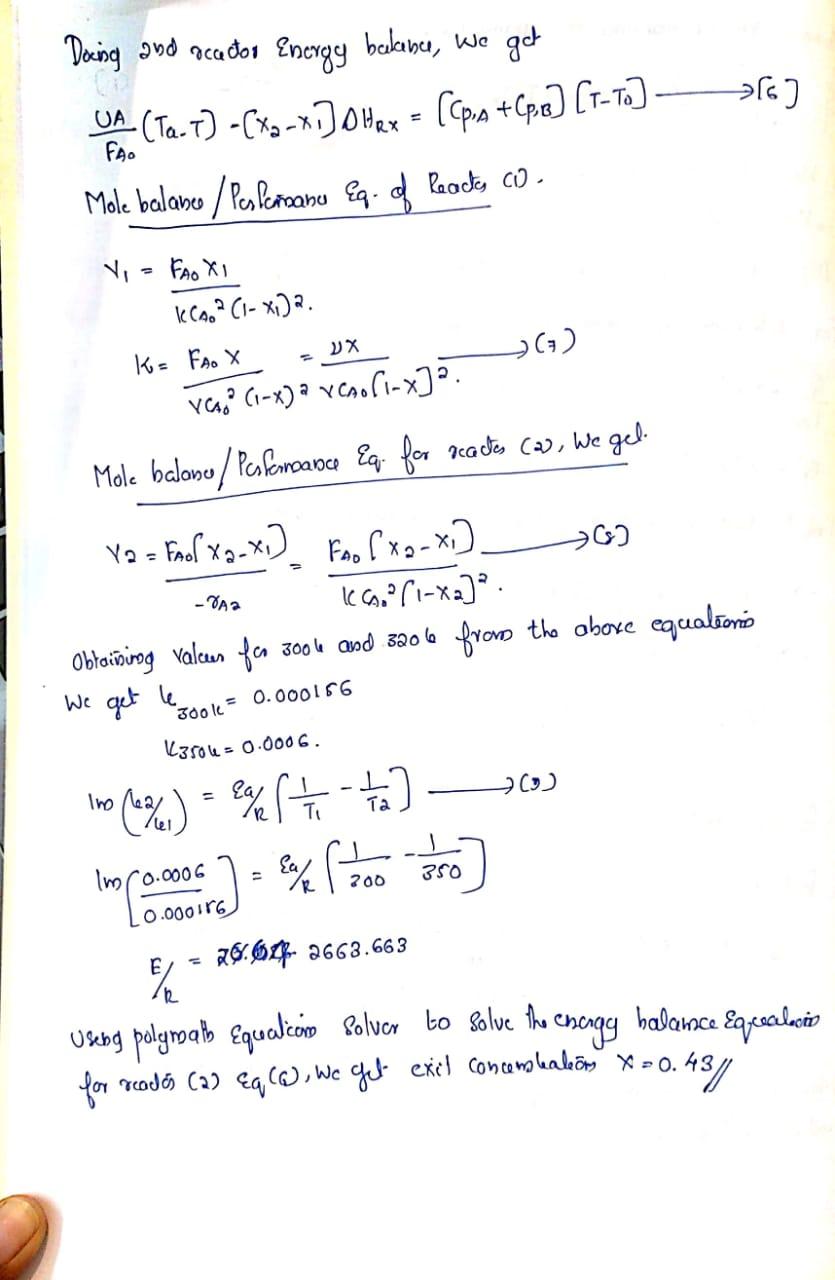
The gas phase reaction A+BC follows an elementary rate law and occurs in a 1m3CSTR. The inlet volumetric flow rate is 0.5m3min1 and the entering concentration of A is 1M. The reaction occurs isothermally at 300K. For an equimolar feed of A and B, a 20% conversion is achieved. When the reaction is carried out adiabatically, the exit temperature is 350K and the conversion is 40%. The heat capacities of A,B, and C are 25,35 , and 60kJ/(molK), respectively and are independent of temperature. It is proposed to add a 2nd CSTR of the same size in series with the first CSTR. There is a heat exchanger attached to the 2nd CSTR with UA =4.0 kJ/(minK), and the coolant fluid enters and exits the heat exchanger at the same temperature of 350K. Assume all reactors operate isobarically. A. What is the rate of heat removal needed for isothermal operation in the first CSTR? B. What is the final conversion at the exit of the second reactor if the first reactor is operated isothermally? C. What would the final conversion be if the second CSTR were replaced with a 1m3 PET with Ua=10(kJ/(m3minK)) and Ta=300K. D. A chemist suggests that the reverse reaction cannot be neglected. From thermodynamics, we know that KCC=2L/mol at 310K. What conversion can be achieved if the entering temperature to the PFR in part C is 300K and Ta=300K ? You may assume that the first CSTR achieves a conversion of 0.2 for the problem. Givied Data Clias phat reaction A+BC \{Elemantarsy rat Daw]. Volume of CSTR =103 The imlet Yolamcric flow nate =0,5ro3/ min +CA0, entereng concembalice of A=1B. + Cobreskion, XA=XB=20% + Temperature (Isolliereal), T=300k. + Upan carveoing out the readion @ adiabalic limpesations T=3506 and adiabatic Converseon, xad=40% CPA=2m/mol6,CP,B=35kJ/moll,CP,C=60kJ/roollC + It is proposed to add a and CSTR iro secics (XA=VB), Ihe and CSTR has a heat exchaness attached, UA=4eJ/memle. Jo Jind (a) Ratc of heat removal needed fos isolticomal opcrationo (b) Final Conversion at the exit of and reactos of the reacter 1 is operated isotticinally (c) Final conversion when and corR is replaced by 103PFT witts Ua=10k/miml,Ta=300k. (d) Chemisl sags reverse readrom portecidely, , lec =2L/mol (a) siolu Ta=300k. What converion can be acheived if the enotering tomperature to the PFR ino (c) is 3006 and a=3004. (c) Whem esir is replaced by 1m3PFR wab AA=T/m3 menble Exproming the chergy balamse in difteremteal farm, we gel. dT/dV=FA0QiCpiUA(TaT)rAHrx=FA0[CpA+CpB]UA(TaT)AHrx]10] rA=6CACB. harou dtdCA=dtdCB=rA Eq(p0) can he solved using polymalls soipt to arrovc at x=0.393, (d) Now asumeby revorse radion pomshely, the apdatio rato law is rA=k[CACBkcCC][11]kC=kC1exp{RHrx{T11T1}}[12] (a) two geven tompuatichs of 300l and 3106 ferodibiog dhod solveriog equation in polymath , we got the convonion as 0.3 (a) Isobermal Encsay halama \&s girem as. FA0QFA0Ws0x[Hkx+Cp(TTk]]=Qi(Ci(10T0)(1) As s=0 [No shatt war] Reation is clonscrotary a=b=1, heroce DCp=Cp,C[Cp,B+Cp,A]=60(35+25)65/roole=0 FAOQ=HRX=0[2]Q=FAOHR=CAOHR[3] To foosd the heat of radion, takenog the adiabatec case, we get xHRx=QiCpi[TT0][4][Q=0,l=0]-xHRX=(CA+CPB)(TT0)C5](DHRX)=0.4[25+35]65/moll[350300]6=7500ks/rool [for adtabatii, conversion is xa=0.4, hance applicd] To food heal romoved in isoltiomnd oparateore, we get: =7.5105ks/rool// Daing and reator Energy bulana, we get FA0UA(TaT)[x2x1]DHRx=[(p,A+Cp,B][TT0][6] Mole bulanee/Pesfermanu Eq. of Reactes (D. V1K=k(A02(1x1)2.FA0X1=VC02(1x)2FA0x=VCAO[1x]2x() Mole balano/Pcifornance q. for ractes (2), We gel. V2=A2FAO[x2x1]=kCA2[1x2]2FA0[x2x1]Cs] Obtaining values for 3006 and 3206 from the above equationis We get l300k=0.000156 k3504=0.0006.lm(62/6)=a/R[T11T21](3)lm[0.00011660.0006]=a/R[20013501]E/R=26.64.2663.663 Useng polymatb Equalicon Solver to solve the enengy halance Equealoio for reades (2) q(Q), we get exil concemleateors x=0.43 The gas phase reaction A+BC follows an elementary rate law and occurs in a 1m3CSTR. The inlet volumetric flow rate is 0.5m3min1 and the entering concentration of A is 1M. The reaction occurs isothermally at 300K. For an equimolar feed of A and B, a 20% conversion is achieved. When the reaction is carried out adiabatically, the exit temperature is 350K and the conversion is 40%. The heat capacities of A,B, and C are 25,35 , and 60kJ/(molK), respectively and are independent of temperature. It is proposed to add a 2nd CSTR of the same size in series with the first CSTR. There is a heat exchanger attached to the 2nd CSTR with UA =4.0 kJ/(minK), and the coolant fluid enters and exits the heat exchanger at the same temperature of 350K. Assume all reactors operate isobarically. A. What is the rate of heat removal needed for isothermal operation in the first CSTR? B. What is the final conversion at the exit of the second reactor if the first reactor is operated isothermally? C. What would the final conversion be if the second CSTR were replaced with a 1m3 PET with Ua=10(kJ/(m3minK)) and Ta=300K. D. A chemist suggests that the reverse reaction cannot be neglected. From thermodynamics, we know that KCC=2L/mol at 310K. What conversion can be achieved if the entering temperature to the PFR in part C is 300K and Ta=300K ? You may assume that the first CSTR achieves a conversion of 0.2 for the problem. Givied Data Clias phat reaction A+BC \{Elemantarsy rat Daw]. Volume of CSTR =103 The imlet Yolamcric flow nate =0,5ro3/ min +CA0, entereng concembalice of A=1B. + Cobreskion, XA=XB=20% + Temperature (Isolliereal), T=300k. + Upan carveoing out the readion @ adiabalic limpesations T=3506 and adiabatic Converseon, xad=40% CPA=2m/mol6,CP,B=35kJ/moll,CP,C=60kJ/roollC + It is proposed to add a and CSTR iro secics (XA=VB), Ihe and CSTR has a heat exchaness attached, UA=4eJ/memle. Jo Jind (a) Ratc of heat removal needed fos isolticomal opcrationo (b) Final Conversion at the exit of and reactos of the reacter 1 is operated isotticinally (c) Final conversion when and corR is replaced by 103PFT witts Ua=10k/miml,Ta=300k. (d) Chemisl sags reverse readrom portecidely, , lec =2L/mol (a) siolu Ta=300k. What converion can be acheived if the enotering tomperature to the PFR ino (c) is 3006 and a=3004. (c) Whem esir is replaced by 1m3PFR wab AA=T/m3 menble Exproming the chergy balamse in difteremteal farm, we gel. dT/dV=FA0QiCpiUA(TaT)rAHrx=FA0[CpA+CpB]UA(TaT)AHrx]10] rA=6CACB. harou dtdCA=dtdCB=rA Eq(p0) can he solved using polymalls soipt to arrovc at x=0.393, (d) Now asumeby revorse radion pomshely, the apdatio rato law is rA=k[CACBkcCC][11]kC=kC1exp{RHrx{T11T1}}[12] (a) two geven tompuatichs of 300l and 3106 ferodibiog dhod solveriog equation in polymath , we got the convonion as 0.3 (a) Isobermal Encsay halama \&s girem as. FA0QFA0Ws0x[Hkx+Cp(TTk]]=Qi(Ci(10T0)(1) As s=0 [No shatt war] Reation is clonscrotary a=b=1, heroce DCp=Cp,C[Cp,B+Cp,A]=60(35+25)65/roole=0 FAOQ=HRX=0[2]Q=FAOHR=CAOHR[3] To foosd the heat of radion, takenog the adiabatec case, we get xHRx=QiCpi[TT0][4][Q=0,l=0]-xHRX=(CA+CPB)(TT0)C5](DHRX)=0.4[25+35]65/moll[350300]6=7500ks/rool [for adtabatii, conversion is xa=0.4, hance applicd] To food heal romoved in isoltiomnd oparateore, we get: =7.5105ks/rool// Daing and reator Energy bulana, we get FA0UA(TaT)[x2x1]DHRx=[(p,A+Cp,B][TT0][6] Mole bulanee/Pesfermanu Eq. of Reactes (D. V1K=k(A02(1x1)2.FA0X1=VC02(1x)2FA0x=VCAO[1x]2x() Mole balano/Pcifornance q. for ractes (2), We gel. V2=A2FAO[x2x1]=kCA2[1x2]2FA0[x2x1]Cs] Obtaining values for 3006 and 3206 from the above equationis We get l300k=0.000156 k3504=0.0006.lm(62/6)=a/R[T11T21](3)lm[0.00011660.0006]=a/R[20013501]E/R=26.64.2663.663 Useng polymatb Equalicon Solver to solve the enengy halance Equealoio for reades (2) q(Q), we get exil concemleateors x=0.43
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


