Question: Data you may need: 1e= 1.6x10-19 C; o (vacuum) = (8.8x10-12)J-4C?m-; (water) = 780; NA=6.02x1023 moll, 1 kcal = 4.184 kJ. = Hydrogen bond is
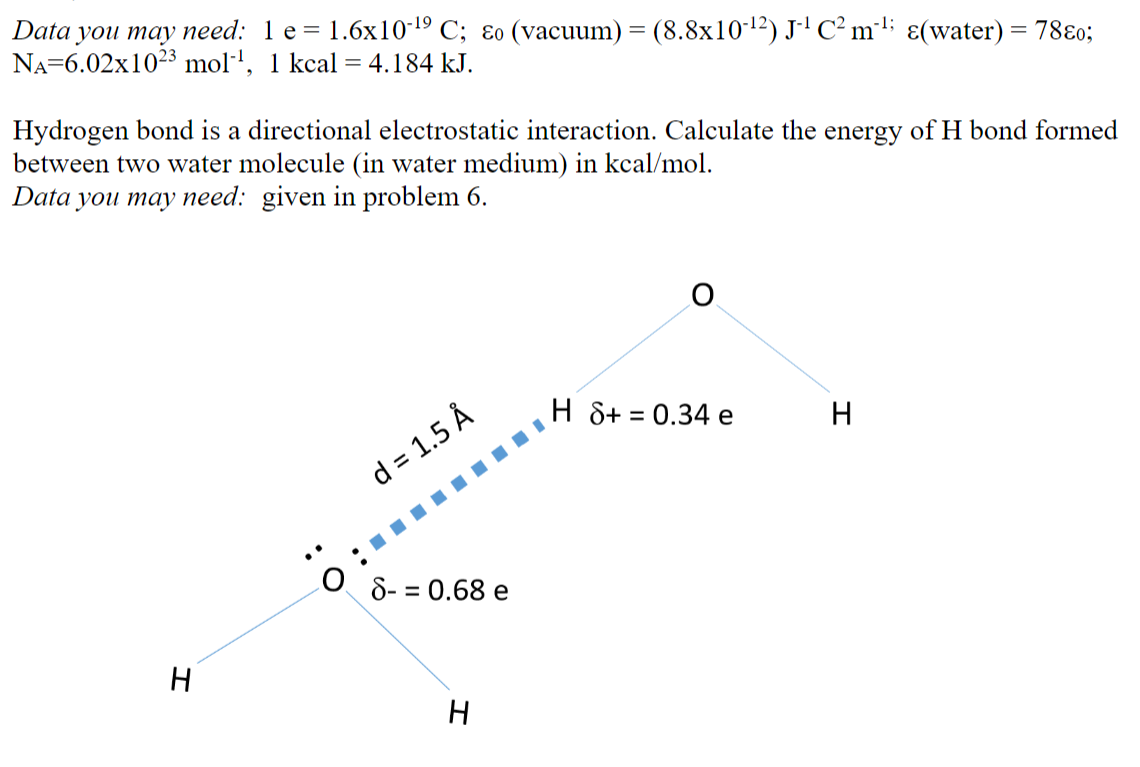
Data you may need: 1e= 1.6x10-19 C; o (vacuum) = (8.8x10-12)J-4C?m-; (water) = 780; NA=6.02x1023 moll, 1 kcal = 4.184 kJ. = Hydrogen bond is a directional electrostatic interaction. Calculate the energy of H bond formed between two water molecule (in water medium) in kcal/mol. Data you may need: given in problem 6. H S+ = 0.34 e = H . d = 1.5 IIII O 8- = 0.68 e = H H
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


