Question: Define the State Postulate. Why is it so important in thermodynamics? ( ii ) Explain why for a compressible substance, the specific heat CP is
Define the State Postulate. Why is it so important in thermodynamics?
ii Explain why for a compressible substance, the specific heat CP is always larger than the
specific heat Cv
iii Explain why for an incompressible substance the internal energy uPT can be accurately
approximated as uT
iv Explain using a sketch of a phase diagram for water P versus T what happens to the melting
and boiling temperatures as a function of the pressure.
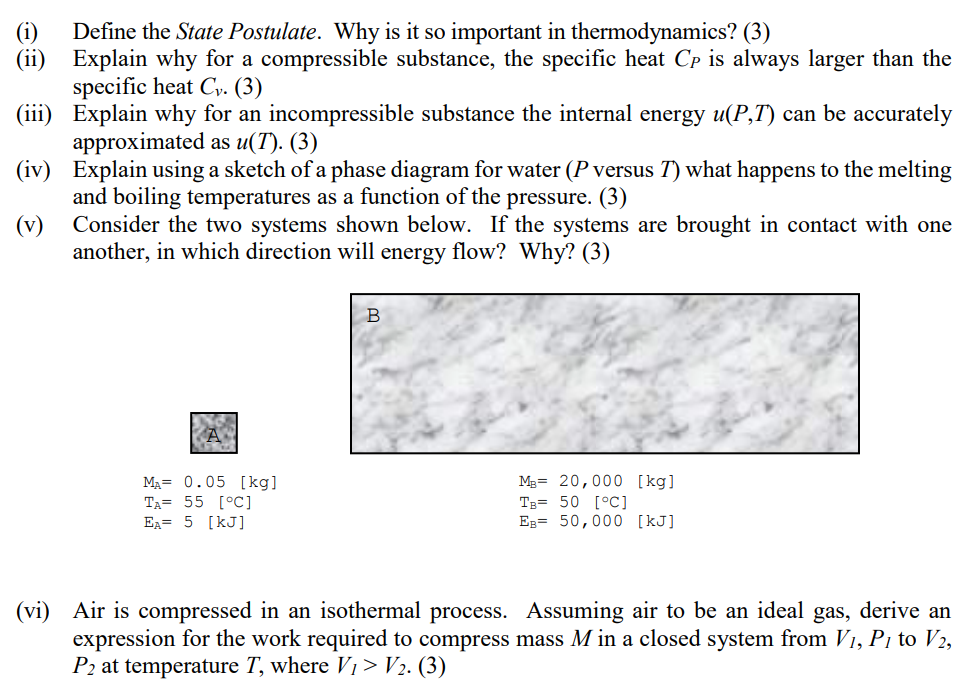
v Consider the two systems shown below. If the systems are brought in contact with one
another, in which direction will energy flow? Why?
vi Air is compressed in an isothermal process. Assuming air to be an ideal gas, derive an
expression for the work required to compress mass M in a closed system from V P to V
P at temperature T where V V
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


