Question: Dehydration is an equilibrium process that involves loss of water from an alcohol. The reverse reaction is hydration of an alkene, which is an addition
Dehydration is an equilibrium process that involves loss of water from an alcohol. The reverse reaction is hydration of an alkene, which is an "addition reaction. This is a reaction that must be driven to completion using Le Chatelier's principle. Examine the equilibrium shown below, how are we driving the equilibrium to form alkene in this reaction?
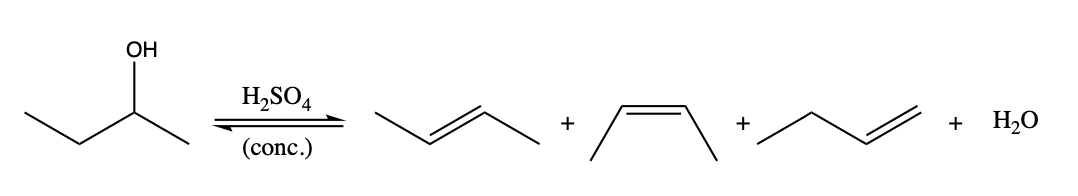
H2SO4 + + + ,0 (conc.)
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


