Question: Derive the rate equation for each different rate controlling assumption For propane dehydrogenation reaction (equation shown below), catalyst such as noble metal Pt supported on
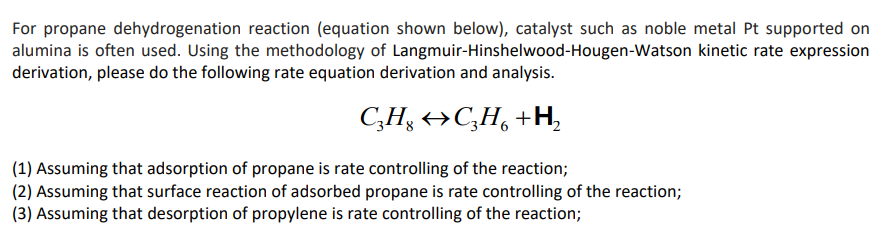
Derive the rate equation for each different rate controlling assumption
For propane dehydrogenation reaction (equation shown below), catalyst such as noble metal Pt supported on alumina is often used. Using the methodology of Langmuir-Hinshelwood-Hougen-Watson kinetic rate expression derivation, please do the following rate equation derivation and analysis. CzH, HC2H6 +H2 (1) Assuming that adsorption of propane is rate controlling of the reaction; (2) Assuming that surface reaction of adsorbed propane is rate controlling of the reaction; (3) Assuming that desorption of propylene is rate controlling of the reaction
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


