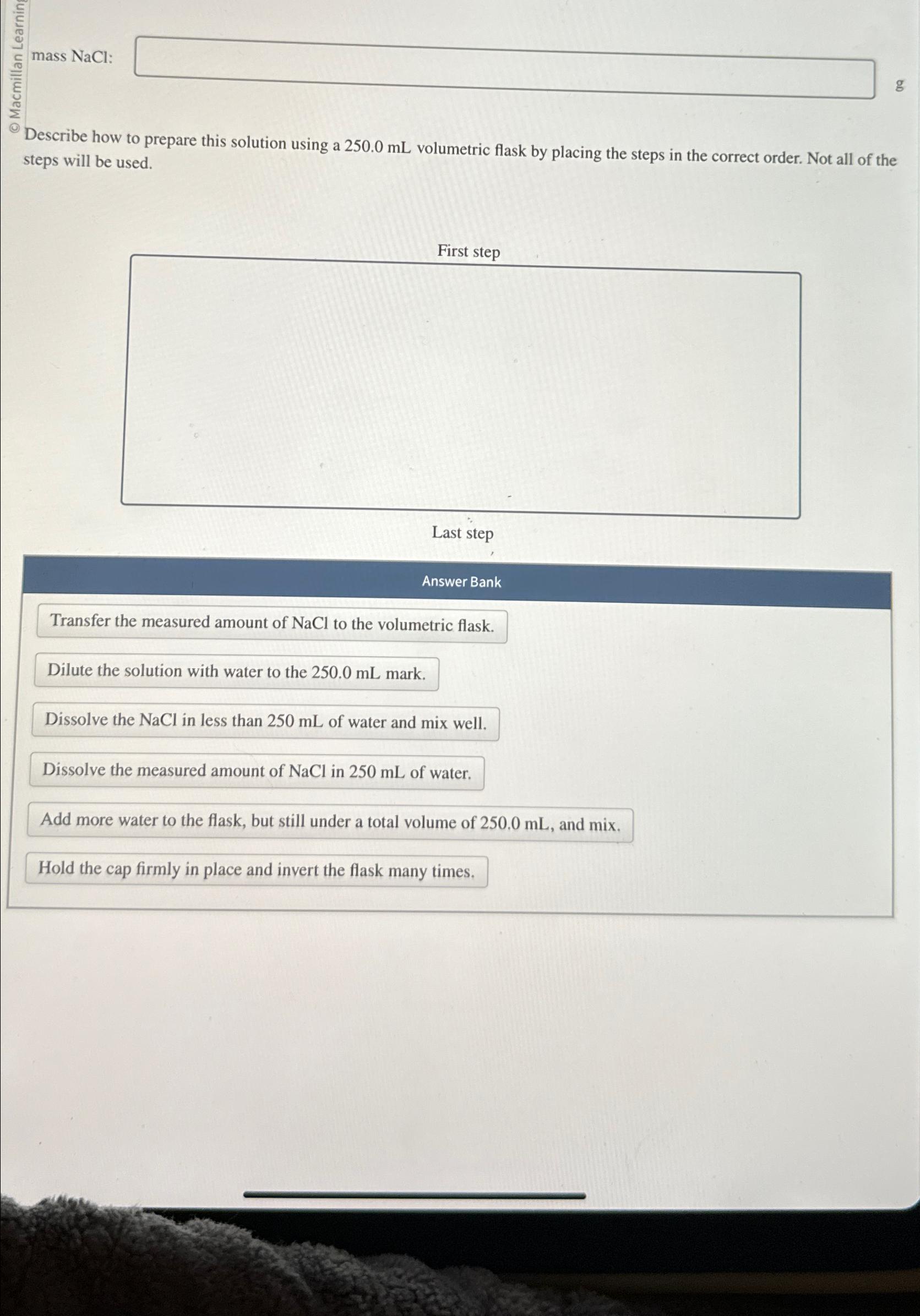
Question: Describe how to prepare this solution using a 250.0mL volumetric flask by placing the steps in the correct order. Not all of the steps will
Describe how to prepare this solution using a
250.0mLvolumetric flask by placing the steps in the correct order. Not all of the steps will be used.\ Last step\ Answer Bank\ Transfer the measured amount of
NaClto the volumetric flask.\ Dilute the solution with water to the
250.0mLmark.\ Dissolve the
NaClin less than
250mLof water and mix well.\ Dissolve the measured amount of
NaClin
250mLof water.\ Add more water to the flask, but still under a total volume of
250.0mL, and mix.\ Hold the cap firmly in place and invert the flask many times.
Describe how to prepare this solution using a 250.0mL volumetric flask by placing the steps in the correct order. Not all of the steps will be used
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


