Question: Despite being able to calculate enthalpy residuals using any EOS with the above equation, this isnt useful due to the difficulty with cubic equations discussed
Despite being able to calculate enthalpy residuals using any EOS with the above equation, this isnt useful due to the difficulty with cubic equations discussed in question 2. However, we can derive the following expression: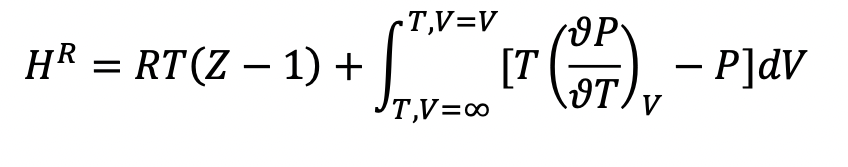
Show the derivation explaining, *clearly* each step and briefly explain why this is better for traditional equations of state.
HR=RT(Z1)+T,V=T,V=V[T(TP)VP]dV
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


