Question: detailed solution step by step Problem 6: Consider an anaerobic fermentation by yeast. The empirical formula of the biomass is CH1.7O0.45N0.15 (Molecular weight =23.0 ).
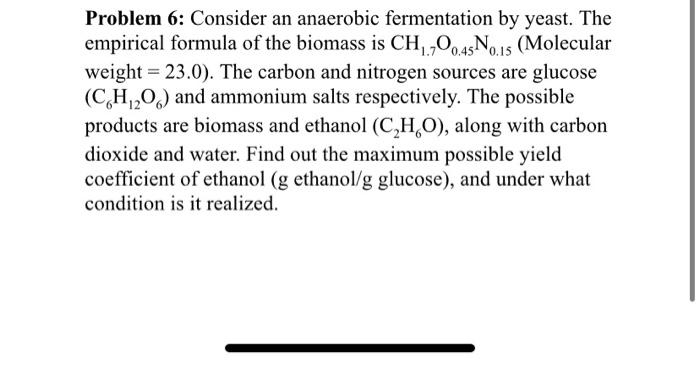
Problem 6: Consider an anaerobic fermentation by yeast. The empirical formula of the biomass is CH1.7O0.45N0.15 (Molecular weight =23.0 ). The carbon and nitrogen sources are glucose (C6H12O6) and ammonium salts respectively. The possible products are biomass and ethanol (C2H6O), along with carbon dioxide and water. Find out the maximum possible yield coefficient of ethanol ( g ethanol /g glucose), and under what condition is it realized
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


