Question: Determine the equilibrium constant, K, for the voltaic cell composed of the following 2 half reactions at 298K. Hg2+(aq)+2eHg(s)Ecello=0.851VCu+(aq)+eCu(s)Ecello=0.521V Report your answer in the format
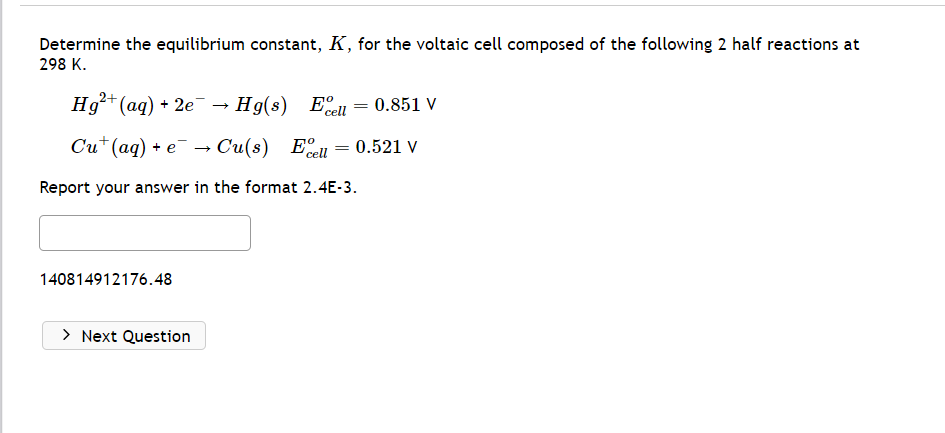
Determine the equilibrium constant, K, for the voltaic cell composed of the following 2 half reactions at 298K. Hg2+(aq)+2eHg(s)Ecello=0.851VCu+(aq)+eCu(s)Ecello=0.521V Report your answer in the format 2.4E-3. 140814912176.48
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


