Question: Determine the equilibrium constant, K, for the voltaic cell composed of the following 2 half reactions at 298 K. Cr3+(aq)+3eCr(s)Ecallo=0.74VCu2+(aq)+2eCu(s)Ecilo=0.342V Report your answer in the
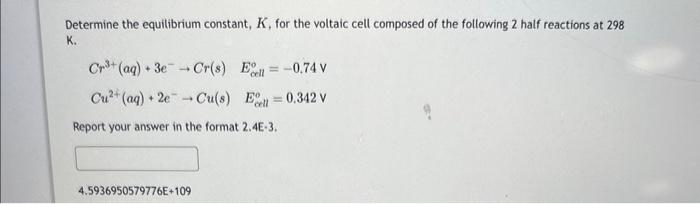
Determine the equilibrium constant, K, for the voltaic cell composed of the following 2 half reactions at 298 K. Cr3+(aq)+3eCr(s)Ecallo=0.74VCu2+(aq)+2eCu(s)Ecilo=0.342V Report your answer in the format 2.4E-3. 4.5936950579776E+109
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


