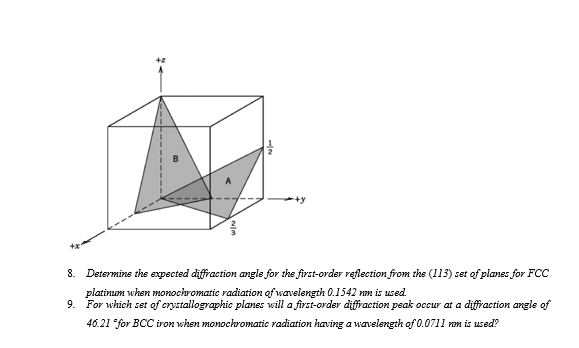
Question: Determine the expected diffraction angle for the first - order reflection from the ( 1 1 3 ) set of planes for FCC platinum when
Determine the expected diffraction angle for the firstorder reflection from the set of planes for FCC platinum when monochromatic radiation of wavelength nm is used.
For which set of crystallographic planes will a firstorder diffraction peak occur at a diffraction angle of for iron when monochromatic radiation having a wavelength of nm is used?
Chromium has four naturallyoccurring isotopes: of with an atomic weight of
of with an atomic weight of of with an
atomic weight of amu and of with an atomic weight of amu On
the basis of these data, confirm that the average atomic weight of Cr is amu
Relative to electrons and electron states, what does each of the four quantum numbers specify?
Give the electron configurations for the following ions: and
Calculate the force of attraction between and an ion the centers of which are
separated by a distance of nm
The force of attraction between a divalent cation and a monovalent anion is If
the ionic radius of the cation is nm what is the anion radius?
For a ion pair, attractive and repulsive energies and respectively, depend on
the distance between the ions according to
For these expressions, energies are expressed in electron volts per Cl pair, and is the
distance in nanometers. The net energy is just the sum of the two expressions above.
a Superimpose on a single plot and versus up to nm
b On the basis of this plot, determine j the equilibrium spacing ro between the and
Clions, and ii the magnitude of the bonding energy between the two ions.
c Mathematically determine the ro and Eo values using the solutions to Problem and
compare these with the graphical results from part b
Compute the percents ionic character of the interatomic bonds for each of the following
compounds: ZnTe, InSb and
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


