Question: Determine the freezing point decrease and boiling point increase after 10.0g of KCl is dissolved in 97.0g of H2O. (use table provided in notes to
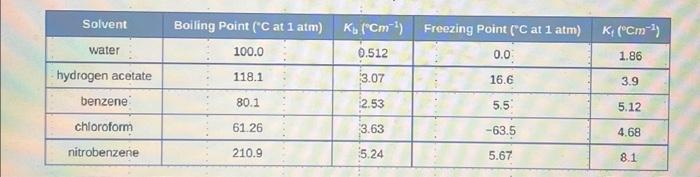
Determine the freezing point decrease and boiling point increase after 10.0g of KCl is dissolved in 97.0g of H2O. (use table provided in notes to determine the solvent K0 and K1 ). \begin{tabular}{|c|c|c|c|c|} \hline Solvent & Boiling Point (C at 1atm) & Kb(Cm1) & Freezing Point (C at 1atm) & Kr(Cm1) \\ \hline water & 100.0 & 0.512 & 0.0 & 1.86 \\ \hline hydrogen acetate & 118.1 & 3.07 & 16.6 & 3.9 \\ \hline benzene: & 80.1 & 2.53 & 5.5 & 5.12 \\ \hline chioroform & 61.26 & 3.63 & 63.5 & 4.68 \\ \hline nitrobenzene & 210.9 & 5.24 & 5.67 & 8.1 \\ \hline \end{tabular}
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


