Question: Determine the oxidation state for each of the elements below. The oxidation state of 15 sulfur in sulfur trioxide SO3 The oxidation state of 15

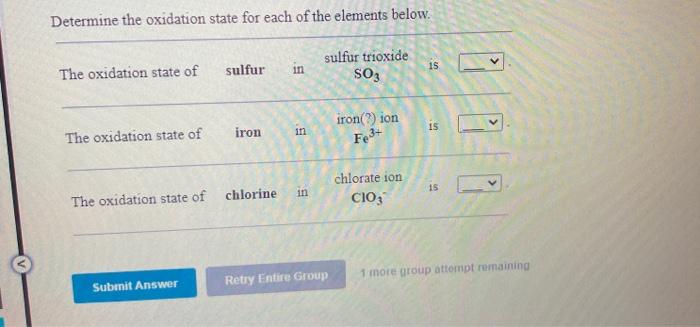
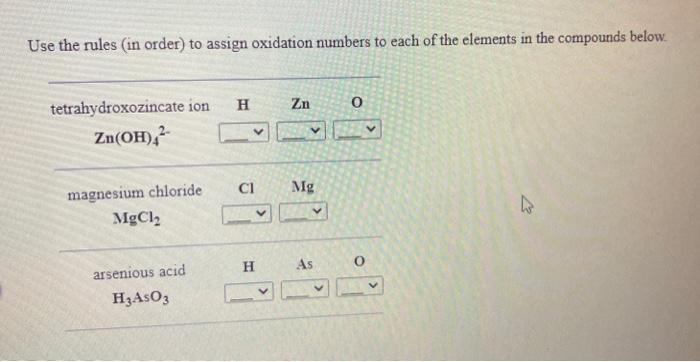
Determine the oxidation state for each of the elements below. The oxidation state of 15 sulfur in sulfur trioxide SO3 The oxidation state of 15 nitrogen in nitrosyl fluoride NOF 15 zinc hydroxide Zn(OH)2 zinc in The oxidation state of Determine the oxidation state for each of the elements below. The oxidation state of sulfur in sulfur trioxide SO3 iron() ion 15 in iron The oxidation state of Fe3+ chlorate ion CIO3 15 The oxidation state of chlorine Submit Answer Retry Entire Group 1 more group attempt remaining Use the rules in order to assign oxidation numbers to each of the elements in the compounds below. H Zn o tetrahydroxozincate ion Zn(OH)2 CI Mg magnesium chloride MgCl2 H As arsenious acid H:AsO 3
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


