Question: Determine the reaction order, the rate constant, and the unitsof the rate constant. For A products, time and concentration data were collected and plotted as
Determine the reaction order, the rate constant, and the unitsof the rate constant.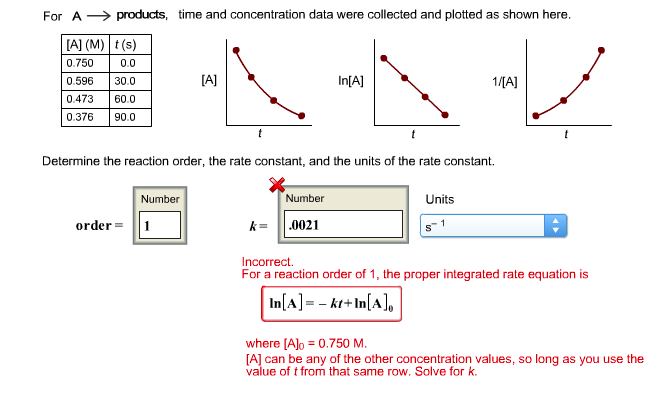
For A products, time and concentration data were collected and plotted as shown here. [A] (M) t(s) 0.750 0.0 0.596 30.0 0.473 60.0 0.376 90.0 Number [A] order = 1 Determine the reaction order, the rate constant, and the units of the rate constant. In[A] Number k= .0021 Units 1 1/[A] S t Incorrect. For a reaction order of 1, the proper integrated rate equation is In[A] = - kt+In [A], where [A]o = 0.750 M. [A] can be any of the other concentration values, so long as you use the value of t from that same row. Solve for k.
Step by Step Solution
3.54 Rating (154 Votes )
There are 3 Steps involved in it
K Let 2303 t log Kc at t ... View full answer

Get step-by-step solutions from verified subject matter experts


