Question: Develop expressions for the specific enthalpy and entropy changes [h(v2, T) - h(v1, 7)], [s(v2, T) - s(v1, T)], using the Redlich-Kwong equation of state.
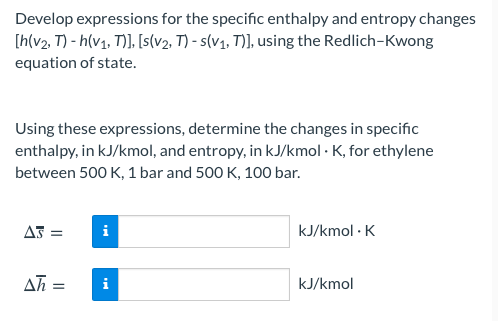
Develop expressions for the specific enthalpy and entropy changes [h(v2, T) - h(v1, 7)], [s(v2, T) - s(v1, T)], using the Redlich-Kwong equation of state. Using these expressions, determine the changes in specific enthalpy, in kJ/kmol, and entropy, in kJ/kmol K, for ethylene between 500 K, 1 bar and 500 K, 100 bar. A5 = = i i kJ/kmol. Ah = = i kJ/kmol
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


